MelatoninMelatonin receptors MT inhibitor CAS# 73-31-4 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 73-31-4 | SDF | Download SDF |
| PubChem ID | 896 | Appearance | Powder |
| Formula | C13H16N2O2 | M.Wt | 232.28 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | N-Acetyl-5-methoxytryptamine | ||
| Solubility | DMSO : 100 mg/mL (430.51 mM; Need ultrasonic) Ethanol : ≥ 50 mg/mL (215.26 mM) *"≥" means soluble, but saturation unknown. | ||
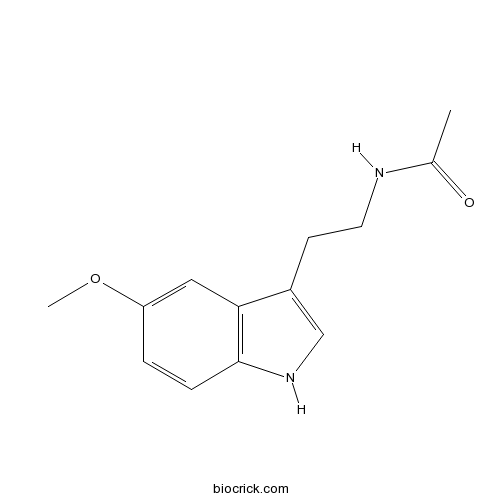
| Chemical Name | N-[2-(5-methoxy-1H-indol-3-yl)ethyl]acetamide | ||
| SMILES | CC(=O)NCCC1=CNC2=C1C=C(C=C2)OC | ||
| Standard InChIKey | DRLFMBDRBRZALE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Melatonin, a hormone produced in the brain, is a potent melatonin receptor activator, and possesses important anti-cancer, antioxidative and anti-inflammatory properties, it can reduce lead toxicity in vivo and in vitro. Melatonin may be useful as a pharmacological agent to protect against hepatic metabolic diseases due to its ability to regulate expression of miR-23a. |
| Targets | Caspase | ERK | CDK |
| In vivo | Melatonin ameliorates ER stress-mediated hepatic steatosis through miR-23a in the liver.[Pubmed: 25660457]Biochem Biophys Res Commun. 2015 Mar 13;458(3):462-9.The endoplasmic reticulum (ER) stress induces hepatic steatosis and inflammation in the liver. Although Melatonin ameliorates ER stress-target genes, it remains unknown whether Melatonin protects against hepatic steatosis as well as inflammation through regulation of miRNA. MicroRNAs have been identified as pivotal regulators in the field of gene regulation and their dysfunctions are a common feature in a variety of metabolic diseases. Especially, among miRNAs, miR-23a has been shown to regulate ER stress.
Melatonin reduces lead levels in blood, brain and bone and increases lead excretion in rats subjected to subacute lead treatment.[Pubmed: 25601058]Toxicol Lett. 2015 Mar 4;233(2):78-83.Melatonin, a hormone known for its effects on free radical scavenging and antioxidant activity, can reduce lead toxicity in vivo and in vitro.We examined the effects of Melatonin on lead bio-distribution.
Melatonin in patients with cancer receiving chemotherapy: a randomized, double-blind, placebo-controlled trial.[Pubmed: 25503168]Anticancer Res. 2014 Dec;34(12):7327-37.The MIRCIT trial was a randomized, double-blind, placebo-controlled study of advanced Non-small cell lung cancer (NSCLC).
|
| Cell Research | Melatonin potentiates cisplatin-induced apoptosis and cell cycle arrest in human lung adenocarcinoma cells.[Pubmed: 25580987]Cell Prolif. 2015 Feb;48(1):67-77.Melatonin produces anti-cancer effects via several mechanisms, including by induction of apoptosis. In this way, it has been shown to be of use, in combination with chemotherapeutic drugs, for cancer treatment. The study described here has evaluated effects of Melatonin on cytotoxicity, apoptosis and cell cycle arrest induced with the chemotherapeutic agent cisplatin, in human lung adenocarcinoma cisplatin-sensitive cell line (SK-LU-1), which previously had only limit data.
|
| Animal Research | Melatonin ameliorates dexamethasone-induced inhibitory effects on the proliferation of cultured progenitor cells obtained from adult rat hippocampus.[Pubmed: 25305353]J Steroid Biochem Mol Biol. 2015 Jan;145:38-48.Glucocorticoids, hormones that are released in response to stress, induce neuronal cell damage. The hippocampus is a primary target of glucocorticoids in the brain, the effects of which include the suppression of cell proliferation and diminished neurogenesis in the dentate gyrus.
Our previous study found that Melatonin, synthesized primarily in the pineal, pretreatment prevented the negative effects of dexamethasone, the glucocorticoid receptor agonist, on behavior and neurogenesis in rat hippocampus.
|

Melatonin Dilution Calculator

Melatonin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3051 mL | 21.5257 mL | 43.0515 mL | 86.103 mL | 107.6287 mL |
| 5 mM | 0.861 mL | 4.3051 mL | 8.6103 mL | 17.2206 mL | 21.5257 mL |
| 10 mM | 0.4305 mL | 2.1526 mL | 4.3051 mL | 8.6103 mL | 10.7629 mL |
| 50 mM | 0.0861 mL | 0.4305 mL | 0.861 mL | 1.7221 mL | 2.1526 mL |
| 100 mM | 0.0431 mL | 0.2153 mL | 0.4305 mL | 0.861 mL | 1.0763 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Endogenous hormone that acts as an agonist at melatonin receptors MT1 and MT2. Exhibits a prominent role in the control of circadian rhythm, displays immunomodulatory activity and acts as a powerful antioxidant in vivo.
- Adenine
Catalog No.:BCC4450
CAS No.:73-24-5
- H-Trp-OH
Catalog No.:BCC3111
CAS No.:73-22-3
- Cordycepin
Catalog No.:BCN5389
CAS No.:73-03-0
- L-(-)-threo-3-Hydroxyaspartic acid
Catalog No.:BCC6565
CAS No.:7298-99-9
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
- BMS-707035
Catalog No.:BCC2133
CAS No.:729607-74-3
- XRP44X
Catalog No.:BCC7568
CAS No.:729605-21-4
- Gomisin O
Catalog No.:BCN2875
CAS No.:72960-22-6
- Gomisin E
Catalog No.:BCN7031
CAS No.:72960-21-5
- 6,7-Dihydroneridienone A
Catalog No.:BCN4020
CAS No.:72959-46-7
- 2-Furoyl-LIGRLO-amide
Catalog No.:BCC3958
CAS No.:729589-58-6
- Carvedilol
Catalog No.:BCC4324
CAS No.:72956-09-3
- H-Ile-OH
Catalog No.:BCC2960
CAS No.:73-32-5
- Guanine
Catalog No.:BCN8414
CAS No.:73-40-5
- Lidocaine hydrochloride
Catalog No.:BCC9009
CAS No.:73-78-9
- 15-Isopimarene-8,18-diol
Catalog No.:BCN4287
CAS No.:73002-86-5
- CyPPA
Catalog No.:BCC7526
CAS No.:73029-73-9
- Atractylenolide III
Catalog No.:BCN1045
CAS No.:73030-71-4
- Epigomisin O
Catalog No.:BCN2862
CAS No.:73036-31-4
- Atractylenolide I
Catalog No.:BCN1043
CAS No.:73069-13-3
- Atractylenolide II
Catalog No.:BCN1044
CAS No.:73069-14-4
- Praeruptorin A
Catalog No.:BCN4987
CAS No.:73069-25-7
- (+)-Praeruptorin A
Catalog No.:BCN4989
CAS No.:73069-27-9
- Praeruptorin D
Catalog No.:BCN4990
CAS No.:73069-28-0
Melatonin in patients with cancer receiving chemotherapy: a randomized, double-blind, placebo-controlled trial.[Pubmed:25503168]
Anticancer Res. 2014 Dec;34(12):7327-37.
BACKGROUND: The MIRCIT trial was a randomized, double-blind, placebo-controlled study of advanced Non-small cell lung cancer (NSCLC). PATIENTS AND METHODS: Patients were randomized to receive 10 mg or 20 mg of Melatonin or placebo. Assessment of health-related quality of life (HRQoL) was completed at baseline, and at 2, 3 and 7 months. Survival and adverse events were collected. DNA damage marker 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodG) was measured during the first three months of chemotherapy. RESULTS: Patients in the Melatonin-treated group had better adjusted HRQoL scores, with a slightly significantly better score (2.69 points, 95% confidence interval (CI)=0.01-5.38, p=0.049) being found in social well-being. Median survival was 7.3 months (95% CI=3.42-11.14) without significant difference. A great amont of DNA damage marker was observed in the placebo-treated group, and this was associated with lower survival (r(2)=-0.656, p=0.02), implying the protective effect of Melatonin in healthy cells. CONCLUSION: Melatonin in combination with chemotherapy did not affect survival and adverse events of advanced patients with NSCLC, but there was a trend for better HRQoL.
Melatonin ameliorates ER stress-mediated hepatic steatosis through miR-23a in the liver.[Pubmed:25660457]
Biochem Biophys Res Commun. 2015 Mar 13;458(3):462-469.
The endoplasmic reticulum (ER) stress induces hepatic steatosis and inflammation in the liver. Although Melatonin ameliorates ER stress-target genes, it remains unknown whether Melatonin protects against hepatic steatosis as well as inflammation through regulation of miRNA. MicroRNAs have been identified as pivotal regulators in the field of gene regulation and their dysfunctions are a common feature in a variety of metabolic diseases. Especially, among miRNAs, miR-23a has been shown to regulate ER stress. Herein, we investigated the crucial roles of Melatonin in hepatic steatosis and inflammation in vivo. Tunicamycin challenge caused increase of hepatic triglyceride and intracellular calcium levels through activation of ER stress, whereas these phenomena were partially disrupted by Melatonin. We also demonstrated that expression of miR-23a stimulated with tunicamycin was rescued by Melatonin treatment, resulting in reduced ER stress in primary hepatocytes. Overall, these results suggest a new function of Melatonin that is involved in ameliorating ER stress-induced hepatic steatosis and inflammation by attenuating miR-23a. Melatonin may be useful as a pharmacological agent to protect against hepatic metabolic diseases due to its ability to regulate expression of miR-23a.
Melatonin reduces lead levels in blood, brain and bone and increases lead excretion in rats subjected to subacute lead treatment.[Pubmed:25601058]
Toxicol Lett. 2015 Mar 4;233(2):78-83.
Melatonin, a hormone known for its effects on free radical scavenging and antioxidant activity, can reduce lead toxicity in vivo and in vitro.We examined the effects of Melatonin on lead bio-distribution. Rats were intraperitoneally injected with lead acetate (10, 15 or 20mg/kg/day) with or without Melatonin (10mg/kg/day) daily for 10 days. In rats intoxicated with the highest lead doses, those treated with Melatonin had lower lead levels in blood and higher levels in urine and feces than those treated with lead alone, suggesting that Melatonin increases lead excretion. To explore the mechanism underlying this effect, we first assessed whether lead/Melatonin complexes were formed directly. Electronic density functional (DFT) calculations showed that a lead/Melatonin complex is energetically feasible; however, UV spectroscopy and NMR analysis showed no evidence of such complexes. Next, we examined the liver mRNA levels of metallothioneins (MT) 1 and 2. Melatonin cotreatment increased the MT2 mRNA expression in the liver of rats that received the highest doses of lead. The potential effects of MTs on the tissue distribution and excretion of lead are not well understood. This is the first report to suggest that Melatonin directly affects lead levels in organisms exposed to subacute lead intoxication.
Melatonin ameliorates dexamethasone-induced inhibitory effects on the proliferation of cultured progenitor cells obtained from adult rat hippocampus.[Pubmed:25305353]
J Steroid Biochem Mol Biol. 2015 Jan;145:38-48.
Glucocorticoids, hormones that are released in response to stress, induce neuronal cell damage. The hippocampus is a primary target of glucocorticoids in the brain, the effects of which include the suppression of cell proliferation and diminished neurogenesis in the dentate gyrus. Our previous study found that Melatonin, synthesized primarily in the pineal, pretreatment prevented the negative effects of dexamethasone, the glucocorticoid receptor agonist, on behavior and neurogenesis in rat hippocampus. In the present study, we attempted to investigate the interrelationship between Melatonin and dexamethasone on the underlying mechanism of neural stem cell proliferation. Addition of dexamethasone to hippocampal progenitor cells from eight-week old rats resulted in a decrease in the number of neurospheres; pretreatment with Melatonin precluded these effects. The immunocytochemical analyses indicated a reduction of Ki67 and nestin-positive cells in the dexamethasone-treated group, which was minimized by Melatonin pretreatment. A reduction of the extracellular signal-regulated kinase 1 and 2 (ERK1/2) phosphorylation and G1-S phase cell cycle regulators cyclin E and CDK2 in dexamethasone-treated progenitor cells were prevented by pretreatment of Melatonin. Moreover, luzindole, a Melatonin receptor antagonist blocked the positive effect of Melatonin whereas RU48, the glucocorticoid receptor antagonist blocked the negative effect of dexamethasone on the number of neurospheres. Moreover, we also found that dexamethasone increased the glucocorticoid receptor protein but decreased the level of MT1 Melatonin receptor, whereas Melatonin increased the level of MT1 Melatonin receptor but decreased the glucocorticoid receptor protein. These suggest the crosstalk and cross regulation between the Melatonin receptor and the glucocorticoid receptor on hippocampal progenitor cell proliferation.
Melatonin potentiates cisplatin-induced apoptosis and cell cycle arrest in human lung adenocarcinoma cells.[Pubmed:25580987]
Cell Prolif. 2015 Feb;48(1):67-77.
OBJECTIVES: Melatonin produces anti-cancer effects via several mechanisms, including by induction of apoptosis. In this way, it has been shown to be of use, in combination with chemotherapeutic drugs, for cancer treatment. The study described here has evaluated effects of Melatonin on cytotoxicity, apoptosis and cell cycle arrest induced with the chemotherapeutic agent cisplatin, in human lung adenocarcinoma cisplatin-sensitive cell line (SK-LU-1), which previously had only limit data. MATERIALS AND METHODS: Cells of the SK-LU-1 line were treated with Melatonin alone at 1-5 mM concentration or cisplatin alone 10-200 muM, for 48 h in culture. Cytotoxicity was measured by MTT reduction assay. Apoptosis induction was detected by annexin V/PI staining using flow cytometric analysis and DAPI nuclear staining. Change in mitochondrial membrane potential (DeltaPsim) was quantified using DiOC6(3) reagent and activities of caspases-3/7 were also investigated. DNA fractions were measured using propidium iodide (PI) staining. RESULTS: Melatonin or cisplatin alone had 50% (IC50 ) cytotoxicity at 5 mM or 34 muM concentrations respectively. Combination of 1 or 2 mM Melatonin and cisplatin significantly augmented cytotoxicity of cisplatin by reducing its IC50 to 11 and 4 muM, respectively. Consistently, combined treatment increased population of apoptotic cells by elevating mitochondrial membrane depolarization, activating caspases-3/7 and inducing cell cycle arrest in the S phase, compared to treatment with cisplatin alone. CONCLUSION: These data demonstrate that Melatonin enhanced cisplatin-induced cytotoxicity and apoptosis in SK-LU-1 lung cancer cells. SK-LU-1 cell population growth inhibition was mediated by cell cycle arrest in the S phase. These findings suggest that Melatonin has the potential to be used for NSCLC treatment in combination with a chemotherapeutic agent such as cisplatin.
Melatonin and melatonergic drugs on sleep: possible mechanisms of action.[Pubmed:19326288]
Int J Neurosci. 2009;119(6):821-46.
Pineal Melatonin is synthesized and secreted in close association with the light/dark cycle. The temporal relationship between the nocturnal rise in Melatonin secretion and the "opening of the sleep gate" (i.e., the increase in sleep propensity at the beginning of the night), coupled with the sleep-promoting effects of exogenous Melatonin, suggest that Melatonin is involved in the regulation of sleep. The sleep-promoting and sleep/wake rhythm regulating effects of Melatonin are attributed to its action on MT(1) and MT(2) Melatonin receptors present in the suprachiasmatic nucleus (SCN) of the hypothalamus. Animal experiments carried out in rats, cats, and monkeys have revealed that Melatonin has the ability to reduce sleep onset time and increase sleep duration. However, clinical studies reveal inconsistent findings, with some of them reporting beneficial effects of Melatonin on sleep, whereas in others only marginal effects are documented. Recently a prolonged-release 2-mg Melatonin preparation (Circadin(TM)) was approved by the European Medicines Agency as a monotherapy for the short-term treatment of primary insomnia in patients who are aged 55 or above. Several Melatonin derivatives have been shown to increase nonrapid eye movement (NREM) in rats and are of potential pharmacological importance. So far only one of these Melatonin derivatives, ramelteon, has received approval from the U.S. Food and Drug Administration to be used as a sleep promoter. Ramelteon is a novel MT(1) and MT(2) melatonergic agonist that has specific effects on Melatonin receptors in the SCN and is effective in promoting sleep in experimental animals such as cats and monkeys. In clinical trials, ramelteon reduced sleep onset latency and promoted sleep in patients with chronic insomnia, including an older adult population. Both Melatonin and ramelteon promote sleep by regulating the sleep/wake rhythm through their actions on Melatonin receptors in the SCN, a unique mechanism of action not shared by any other hypnotics. Moreover, unlike benzodiazepines, ramelteon causes neither withdrawal effects nor dependence. Agomelatine, another novel melatonergic antidepressant in its final phase of approval for clinical use, has been shown to improve sleep in depressed patients and to have an antidepressant efficacy that is partially attributed to its effects on sleep-regulating mechanisms.
Thirty four years since the discovery of gastrointestinal melatonin.[Pubmed:18812627]
J Physiol Pharmacol. 2008 Aug;59 Suppl 2:33-51.
After the discovery of Melatonin in the pineal gland by Lerner and co-workers in 1958, Melatonin was also detected in the retina and the human appendix. Later, Melatonin was confirmed immunohistologically in all segments of the gastrointestinal tract (GIT), in the guts of bovine embryos and in the GIT of low vertebrates. Melatonin was also confirmed in the pancreas and the hepatobiliary system. Melatonin is produced in the enteroendocrine cells of the GIT mucosa. The concentrations of Melatonin in the GIT are 10-100x higher than in the plasma and the total amount of Melatonin in the GIT is around 400x higher than the amount of Melatonin in the pineal gland. Similar to pineal Melatonin, GIT Melatonin is a multifunctional compound which exhibits some general as well as some specific effects, depending on the organ and the location of GIT tissue. In the GIT, Melatonin exhibits endocrine, paracrine, autocrine and luminal actions. Generally, the episodic secretion of Melatonin from the GIT is related to the intake and digestion of food and to the prevention of tissue damage caused by hydrochloric acid and digestive enzymes. Some actions, such as the scavenging of hydroxyl free radicals, immunoenhancement and antioxidant effects are of general nature, whereas others, such as an increase of mucosal blood flow, the reduction of peristalsis and the regulation of fecal water content, are specific to the tubular GIT. Generally, Melatonin actions oppose those of serotonin. Laboratory and clinical studies indicate that the utilization of Melatonin can prevent or treat pathological conditions such as esophageal and gastric ulcers, pancreatitis, colitis, irritable bowel disease, and colon cancer.
Melatonin as a major skin protectant: from free radical scavenging to DNA damage repair.[Pubmed:18643846]
Exp Dermatol. 2008 Sep;17(9):713-30.
Melatonin, one of the evolutionarily most ancient, highly conserved and most pleiotropic hormones still operative in man, couples complex tissue functions to defined changes in the environment. Showing photoperiod-associated changes in its activity levels in mammals, Melatonin regulates, chronobiological and reproductive systems, coat phenotype and mammary gland functions. However, this chief secretory product of the pineal gland is now recognized to also exert numerous additional functions which range from free radical scavenging and DNA repair via immunomodulation, body weight control and the promotion of wound healing to the coupling of environmental cues to circadian clock gene expression and the modulation of secondary endocrine signalling (e.g. prolactin release, oestrogen receptor-mediated signalling). Some of these activities are mediated by high-affinity membrane (MT1, MT2) or specific cytosolic (MT3/NQO2) and nuclear hormone receptors (ROR alpha), while others reflect receptor-independent antioxidant activities of Melatonin. Recently, it was shown that mammalian (including human) skin and hair follicles are not only Melatonin targets, but also sites of extrapineal Melatonin synthesis. Therefore, we provide here an update of the relevant cutaneous effects and mechanisms of Melatonin, portray Melatonin as a major skin protectant and sketch how its multi-facetted functions may impact on skin biology and pathology. This is illustrated by focussing on recent findings on the role of Melatonin in photodermatology and hair follicle biology. After listing a number of key open questions, we conclude by defining particularly important, clinically relevant perspectives for how Melatonin may become therapeutically exploitable in cutaneous medicine.


