Gomisin JCAS# 66280-25-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 66280-25-9 | SDF | Download SDF |
| PubChem ID | 3001686 | Appearance | Powder |
| Formula | C22H28O6 | M.Wt | 388.5 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
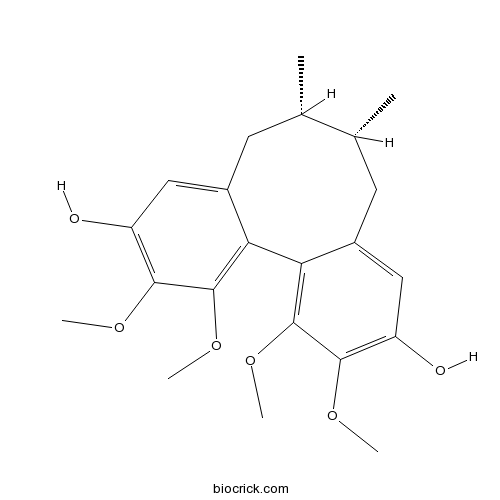
| SMILES | CC1CC2=CC(=C(C(=C2C3=C(C(=C(C=C3CC1C)O)OC)OC)OC)OC)O | ||
| Standard InChIKey | PICOUNAPKDEPCA-TXEJJXNPSA-N | ||
| Standard InChI | InChI=1S/C22H28O6/c1-11-7-13-9-15(23)19(25-3)21(27-5)17(13)18-14(8-12(11)2)10-16(24)20(26-4)22(18)28-6/h9-12,23-24H,7-8H2,1-6H3/t11-,12+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Gomisin J is a good substrate of cytochrome P450 3A4(CYP3A4), it has vasodilatory, anti-inflammatory, anti-diabetes, anti-oxidant, and anti-cancer effects, it also has preventive effects on angiotensin II-induced hypertension via an increased nitric oxide bioavailability. Gomisin J has potential benefits in treating nonalcoholic fatty liver disease, it can suppress lipid accumulation by regulating the expression of lipogenic and lipolytic enzymes and inflammatory molecule. Halogenated gomisin J is a potent inhibitor of the cytopathic effects of human immunodeficiency virus type 1 (HIV-1) on MT-4 human T cells (50% effective dose, 0.1 to 0.5 microM). |
| Targets | NO | ROS | NOS | PI3K | Akt | ERK | JNK | p38MAPK | HIV | NADPH-oxidase | Calcium Channel | AMPK | P450 (e.g. CYP17) |
| In vitro | Gomisin J from Schisandra chinensis induces vascular relaxation via activation of endothelial nitric oxide synthase.[Pubmed: 22728282]Vascul Pharmacol. 2012 Sep-Oct;57(2-4):124-30.Gomisin J (GJ) is a lignan contained in Schisandra chinensis (SC) which is a well-known medicinal herb for improvement of cardiovascular symptoms in Korean. Thus, the present study examined the vascular effects of GJ, and also determined the mechanisms involved. Anti-human immunodeficiency virus (HIV) activities of halogenated gomisin J derivatives, new nonnucleoside inhibitors of HIV type 1 reverse transcriptase.[Pubmed: 8540706]Antimicrob Agents Chemother. 1995 Sep;39(9):2000-7.Halogenated Gomisin J (a derivative of lignan compound), represented by the bromine derivative 1506 [(6R, 7S, S-biar)-4,9-dibromo-3,10-dihydroxy-1,2,11,12-tetramethoxy-6, 7-dimethyl-5,6,7,8- tetrahydrodibenzo[a,c]cyclo-octene], was found to be a potent inhibitor of the cytopathic effects of human immunodeficiency virus type 1 (HIV-1) on MT-4 human T cells (50% effective dose, 0.1 to 0.5 microM).
Anti-lipid peroxidation of gomisin J on liver mitochondria and cultured myocardial cells.[Pubmed: 9863151]Zhongguo Yao Li Xue Bao. 1996 Nov;17(6):538-41.To study the influences of Gomisin J on lipid peroxidation and calcium paradox.
Gomisin J with protective effect against t-BHP-induced oxidative damage in HT22 cells from Schizandra chinensis.[Reference: WebLink]Nat. Prod. Sci., 2006, 12(3):134-7.
Four lignan compounds including Gomisin J (1), schizandrin (2), gomisin A (3), and angeloyl gomisin H (4) have been isolated from the MeOH extract of Schizandra chinensis fruits. |
| In vivo | Preventive effect of gomisin J from Schisandra chinensis on angiotensin II-induced hypertension via an increased nitric oxide bioavailability.[Pubmed: 25427681]Hypertens Res. 2015 Mar;38(3):169-77.Gomisin J (GJ) is a small molecular weight lignan found in Schisandra chinensis and has been demonstrated to have vasodilatory activity. |
| Kinase Assay | Species-dependent drug-drug interaction between irinotecan and anti-diabetes drug Gomisin J.[Reference: WebLink]Molecular docking to understand the interaction between anti-tumor drug candidate gomisin J and cytochrome P450 3A4.[Reference: WebLink]Gomisin J Inhibits Oleic Acid-Induced Hepatic Lipogenesis by Activation of the AMPK-Dependent Pathway and Inhibition of the Hepatokine Fetuin-A in HepG2 Cells.[Pubmed: 26455261]J Agric Food Chem. 2015 Nov 11;63(44):9729-39.The aim of our study is to investigate the molecular mechanism of Gomisin J from Schisandra chinensis on the oleic acid (OA)-induced lipid accumulation in HepG2 cells. Lat. Am. J. Pharm., 2015, 34(2):416-8.
Protein preparation wizard in the Schrödinger suite of programs was employed to process the structure, and the missing residues and hydrogen atoms were added and assigned. Lat. Am. J. Pharm., 2015, 34(4):827-30.
|
| Cell Research | Anti-inflammatory effects of gomisin N, gomisin J, and schisandrin C isolated from the fruit of Schisandra chinensis.[Pubmed: 20139628]Biosci Biotechnol Biochem. 2010;74(2):285-91.Schiandra chinensis is a well-known Chinese traditional medicine for the treatment of hepatic disease. |

Gomisin J Dilution Calculator

Gomisin J Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.574 mL | 12.87 mL | 25.74 mL | 51.4801 mL | 64.3501 mL |
| 5 mM | 0.5148 mL | 2.574 mL | 5.148 mL | 10.296 mL | 12.87 mL |
| 10 mM | 0.2574 mL | 1.287 mL | 2.574 mL | 5.148 mL | 6.435 mL |
| 50 mM | 0.0515 mL | 0.2574 mL | 0.5148 mL | 1.0296 mL | 1.287 mL |
| 100 mM | 0.0257 mL | 0.1287 mL | 0.2574 mL | 0.5148 mL | 0.6435 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Amino-2-methylquinoline
Catalog No.:BCC8677
CAS No.:6628-04-2
- 6'-O-Acetylpaniculoside II
Catalog No.:BCC8316
CAS No.:
- Stemonidine
Catalog No.:BCC8253
CAS No.:66267-46-7
- N-dotriacontanol
Catalog No.:BCC8218
CAS No.:6624-79-9
- N-Benzylanthranilic acid
Catalog No.:BCC9093
CAS No.:6622-55-5
- Cyromazine
Catalog No.:BCC5328
CAS No.:66215-27-8
- Deacetylxylopic acid
Catalog No.:BCN4210
CAS No.:6619-95-0
- Malic acid 4-Me ester
Catalog No.:BCN4209
CAS No.:66178-02-7
- Cimicifugoside
Catalog No.:BCN2623
CAS No.:66176-93-0
- Chromolaenide
Catalog No.:BCN7341
CAS No.:66148-25-2
- Levalbuterol tartrate
Catalog No.:BCC4217
CAS No.:661464-94-4
- 14,17-Epidioxy-28-nor-15-taraxerene-2,3-diol
Catalog No.:BCN1386
CAS No.:66107-60-6
- Pregomisin
Catalog No.:BCN7000
CAS No.:66280-26-0
- 3H-1,2-Benzodithiol-3-one-1,1-dioxide
Catalog No.:BCC8633
CAS No.:66304-01-6
- ML 171
Catalog No.:BCC6252
CAS No.:6631-94-3
- Dihydroguaiaretic acid
Catalog No.:BCN4212
CAS No.:66322-34-7
- 5'-Deoxy-5-fluorocytidine
Catalog No.:BCC8746
CAS No.:66335-38-4
- Ranitidine
Catalog No.:BCC9134
CAS No.:66357-35-5
- Ranitidine Hydrochloride
Catalog No.:BCC4533
CAS No.:66357-59-3
- TGX-221
Catalog No.:BCC1244
CAS No.:663619-89-4
- Methylsyringol
Catalog No.:BCN3535
CAS No.:6638-05-7
- (+)-Apogossypol
Catalog No.:BCC5585
CAS No.:66389-74-0
- Chloranthalactone A
Catalog No.:BCN8022
CAS No.:66395-02-6
- Chloranthalactone B
Catalog No.:BCN8020
CAS No.:66395-03-7
Gomisin J Inhibits Oleic Acid-Induced Hepatic Lipogenesis by Activation of the AMPK-Dependent Pathway and Inhibition of the Hepatokine Fetuin-A in HepG2 Cells.[Pubmed:26455261]
J Agric Food Chem. 2015 Nov 11;63(44):9729-39.
The aim of our study is to investigate the molecular mechanism of Gomisin J from Schisandra chinensis on the oleic acid (OA)-induced lipid accumulation in HepG2 cells. Gomisin J attenuated lipid accumulation in OA-induced HepG2 cells. It also suppressed the expression of lipogenic enzymes and inflammatory mediators and increased the expression of lipolytic enzymes in OA-induced HepG2 cells. Furthermore, the use of specific inhibitors and fetuin-A siRNA and liver kinase B1 (LKB1) siRNA transfected cells demonstrated that Gomisin J regulated lipogenesis and lipolysis via inhibition of fetuin-A and activation of an AMP-activated protein kinase (AMPK)-dependent pathway in HepG2 cells. Our results showed that Gomisin J suppressed lipid accumulation by regulating the expression of lipogenic and lipolytic enzymes and inflammatory molecules through activation of AMPK, LKB1, and Ca(2+)/calmodulin-dependent protein kinase II and inhibition of fetuin-A in HepG2 cells. This suggested that Gomisin J has potential benefits in treating nonalcoholic fatty liver disease.
Gomisin J from Schisandra chinensis induces vascular relaxation via activation of endothelial nitric oxide synthase.[Pubmed:22728282]
Vascul Pharmacol. 2012 Sep-Oct;57(2-4):124-30.
Gomisin J (GJ) is a lignan contained in Schisandra chinensis (SC) which is a well-known medicinal herb for improvement of cardiovascular symptoms in Korean. Thus, the present study examined the vascular effects of GJ, and also determined the mechanisms involved. Exposure of rat thoracic aorta to GJ (1-30mug/ml) resulted in a concentration-dependent vasorelaxation, which was more prominent in the endothelium (ED)-intact aorta. ED-dependent relaxation induced by GJ was markedly attenuated by pretreatment with L-NAME, a nitric oxide synthase (NOS) inhibitor. In the intact endothelial cells of rat thoracic aorta, GJ also enhanced nitric oxide (NO) production. In studies using human coronary artery endothelial cells, GJ enhanced phosphorylation of endothelial NOS (eNOS) at Ser(1177) with increased cytosolic translocation of eNOS, and subsequently increased NO production. These effects of GJ were attenuated not only by calcium chelators including EGTA and BAPTA-AM, but also by LY294002, a PI3K/Akt inhibitor, indicating calcium- and PI3K/Akt-dependent activation of eNOS by GJ. Moreover, the levels of intracellular calcium were increased immediately after GJ administration, but Akt phosphorylation was started to increase at 20min of GJ treatment. Based on these results with the facts that ED-dependent relaxation occurred rapidly after GJ treatment, it was suggested that the ED-dependent vasorelaxant effects of GJ were mediated mainly by calcium-dependent activation of eNOS with subsequent production of endothelial NO.
Anti-lipid peroxidation of gomisin J on liver mitochondria and cultured myocardial cells.[Pubmed:9863151]
Zhongguo Yao Li Xue Bao. 1996 Nov;17(6):538-41.
AIM: To study the influences of Gomisin J on lipid peroxidation and calcium paradox. METHODS: Using two in vitro models of rat liver mitochondria membrane lipid peroxidation (LPO) and cultured myocardial cells. RESULTS: Gomisin J inhibited Fe2+/ascorbic acid and ADP/NADPH-induced LPO with IC50 (95% confidence limits) 5.5 (4.5-6.7) and 4.7 (2.8-7.8) mumol.L-1, respectively, when cultured myocardial cells preincubated with Ca(2+)-free medium for 2 min were incubated with normal medium containing Ca2+, a marked increase of malondialdehyde (MDA) formation occurred and Gomisin J 10 mumol.L-1 protected myocardial cells through decreasing MDA formation. CONCLUSION: Gomisin J inhibits LPO in rat liver mitochondria and protects cultured myocardial cells from being injured by calcium paradox.
Preventive effect of gomisin J from Schisandra chinensis on angiotensin II-induced hypertension via an increased nitric oxide bioavailability.[Pubmed:25427681]
Hypertens Res. 2015 Mar;38(3):169-77.
Gomisin J (GJ) is a small molecular weight lignan found in Schisandra chinensis and has been demonstrated to have vasodilatory activity. In this study, the authors investigated the effect of GJ on blood pressure (BP) in angiotensin II (Ang II)-induced hypertensive mice. In addition, we determined the relative potencies of gomisin A (GA) and GJ with respect to vasodilatory activity and antihypertensive effects. C57/BL6 mice infused s.c. with Ang II (2 mug kg(-1) min(-1) for 2 weeks) showed an increase in BP and a decrease in plasma nitric oxide (NO) metabolites. In the thoracic aortas of Ang II-induced hypertensive mice, a decrease in vascular NO was accompanied by an increase in reactive oxygen species (ROS) production. Furthermore, these alterations in BP, plasma concentrations of NO metabolites and in the vascular productions of NO and ROS in Ang II-treated mice were reversed by the co-administration of GJ (1 and 3 mug kg(-1) min(-1)). In in vitro studies, Ang II decreased the cellular concentration of NO, which was accompanied by a reduction in phosphorylated endothelial nitric oxide synthase (eNOS) and an increase in ROS production. These eNOS phosphorylation and ROS production changes in Ang II-treated cells were also reversed by GJ pretreatment (0-3 mug ml(-1)). Interestingly, the vasodilatory and antihypertensive effects of GJ were more prominent than those of GA. Collectively, an increase in BP in mice treated with Ang II was markedly attenuated by GJ, which was attributed to the preservations of vascular NO bioavailability and eNOS function, and to the inhibition of ROS production in Ang II-induced hypertensive mice.
Anti-human immunodeficiency virus (HIV) activities of halogenated gomisin J derivatives, new nonnucleoside inhibitors of HIV type 1 reverse transcriptase.[Pubmed:8540706]
Antimicrob Agents Chemother. 1995 Sep;39(9):2000-7.
Halogenated Gomisin J (a derivative of lignan compound), represented by the bromine derivative 1506 [(6R, 7S, S-biar)-4,9-dibromo-3,10-dihydroxy-1,2,11,12-tetramethoxy-6, 7-dimethyl-5,6,7,8- tetrahydrodibenzo[a,c]cyclo-octene], was found to be a potent inhibitor of the cytopathic effects of human immunodeficiency virus type 1 (HIV-1) on MT-4 human T cells (50% effective dose, 0.1 to 0.5 microM). Gomisin J derivatives were active in preventing p24 production from acutely HIV-1-infected H9 cells. The selective indices (toxic dose/effective dose) of these compounds were as high as > 300 in some systems. 1506 was active against 3'-azido-3'-deoxythymidine-resistant HIV-1 and acted synergistically with AZT and 2',3'-ddC. 1506 inhibited HIV-1 reverse transcriptase (RT) in vitro but not HIV-1 protease. From the time-of-addition experiment, 1506 was found to inhibit the early phase of the HIV life cycle. A 1506-resistant HIV mutant was selected and shown to possess a mutation within the RT-coding region (at position 188 [Tyr to Leu]). The mutant RT expressed in Escherichia coli was resistant to 1506 in the in vitro RT assay. Some of the HIV strains resistant to other nonnucleoside HIV-1 RT inhibitors were also resistant to 1506. Comparison of various Gomisin J derivatives with Gomisin J showed that iodine, bromine, and chlorine in the fourth and ninth positions increased RT inhibitory activity as well as cytoprotective activity.
Anti-inflammatory effects of gomisin N, gomisin J, and schisandrin C isolated from the fruit of Schisandra chinensis.[Pubmed:20139628]
Biosci Biotechnol Biochem. 2010;74(2):285-91.
Schiandra chinensis is a well-known Chinese traditional medicine for the treatment of hepatic disease. In this study, we investigated whether the nine major compounds of Schiandra chinensis could be applied to suppress lipopolysaccharide (LPS)-induced inflammatory responses in murine macrophages (Raw 264.7 cells). Among the nine lignans, three, Gomisin J, gomisin N, and schisandrin C, were found to reduce nitric oxide (NO) production from LPS-stimulated Raw 264.7 cells. These three lignans showed low cytotoxic effects in Raw 264.7 cells. Pre-treatment of Raw 264.7 cells with Gomisin J, gomisin N, or schisandrin C reduced the expression of mRNA and the secretion of pro-inflammatory cytokines. These inhibitory effects were found to be caused by blockage of p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinases 1 and 2 (ERK 1/2), and c-Jun N-terminal kinase (JNK) phosphorylation.


