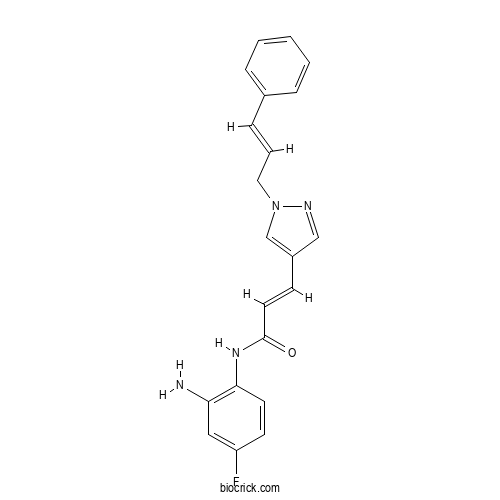
(E)-N-(2-Amino-4-fluorophenyl)-3-(1-cinnamyl-1H-pyrazol-4-yl)acrylamideCAS# 1396841-57-8 |
- RGFP966
Catalog No.:BCC3991
CAS No.:1357389-11-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1396841-57-8 | SDF | Download SDF |
| PubChem ID | 56650312 | Appearance | Powder |
| Formula | C21H19FN4O | M.Wt | 362.4 |
| Type of Compound | Inhibitors | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-N-(2-amino-4-fluorophenyl)-3-[1-[(E)-3-phenylprop-2-enyl]pyrazol-4-yl]prop-2-enamide | ||
| SMILES | C1=CC=C(C=C1)C=CCN2C=C(C=N2)C=CC(=O)NC3=C(C=C(C=C3)F)N | ||
| Standard InChIKey | BLVQHYHDYFTPDV-VCABWLAWSA-N | ||
| Standard InChI | InChI=1S/C21H19FN4O/c22-18-9-10-20(19(23)13-18)25-21(27)11-8-17-14-24-26(15-17)12-4-7-16-5-2-1-3-6-16/h1-11,13-15H,12,23H2,(H,25,27)/b7-4+,11-8+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

(E)-N-(2-Amino-4-fluorophenyl)-3-(1-cinnamyl-1H-pyrazol-4-yl)acrylamide Dilution Calculator

(E)-N-(2-Amino-4-fluorophenyl)-3-(1-cinnamyl-1H-pyrazol-4-yl)acrylamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7594 mL | 13.7969 mL | 27.5938 mL | 55.1876 mL | 68.9845 mL |
| 5 mM | 0.5519 mL | 2.7594 mL | 5.5188 mL | 11.0375 mL | 13.7969 mL |
| 10 mM | 0.2759 mL | 1.3797 mL | 2.7594 mL | 5.5188 mL | 6.8985 mL |
| 50 mM | 0.0552 mL | 0.2759 mL | 0.5519 mL | 1.1038 mL | 1.3797 mL |
| 100 mM | 0.0276 mL | 0.138 mL | 0.2759 mL | 0.5519 mL | 0.6898 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-Hydroxy-3-methoxybenzaldehyde
Catalog No.:BCN0157
CAS No.:148-53-8
- Lappaol B
Catalog No.:BCN0156
CAS No.:62359-60-8
- N-trans-caffeoyltyramine
Catalog No.:BCN0155
CAS No.:103188-48-3
- Helenien
Catalog No.:BCN0154
CAS No.:547-17-1
- Comanthosid B
Catalog No.:BCN0153
CAS No.:70938-60-2
- Candicine
Catalog No.:BCN0151
CAS No.:6656-13-9
- Maritimein
Catalog No.:BCN0150
CAS No.:490-54-0
- 3',4',7-Trihydroxyisoflavone
Catalog No.:BCN0149
CAS No.:485-63-2
- 2'-Hydroxyflavanone
Catalog No.:BCN0148
CAS No.:17348-76-4
- 2',6'-Dihydroxy 4',4-dimethoxydihydrochalcone
Catalog No.:BCN0147
CAS No.:35241-54-4
- Daphnin
Catalog No.:BCN0146
CAS No.:486-55-5
- 3',4',7,8-Tetrahydroxyflavone
Catalog No.:BCN0145
CAS No.:3440-24-2
- Bicyclo[5.3.0]decapentaene
Catalog No.:BCN0159
CAS No.:275-51-4
- 2,5-Dimethoxybenzoic acid
Catalog No.:BCN0160
CAS No.:2785-98-0
- 2,3-Dimethylanthraquinone
Catalog No.:BCN0161
CAS No.:6531-35-7
- Qingdainone
Catalog No.:BCN0162
CAS No.:97457-31-3
- 7-Hydroxy-4'-methoxyflavone
Catalog No.:BCN0163
CAS No.:487-24-1
- 5-Hydroxyflavone
Catalog No.:BCN0164
CAS No.:491-78-1
- Yohimbic acid monohydrate
Catalog No.:BCN0165
CAS No.:522-87-2
- Ethyl isovalerate
Catalog No.:BCN0166
CAS No.:108-64-5
- 2,3-Dihydroxybenzoic acid
Catalog No.:BCN0167
CAS No.:303-38-8
- trans-Chrysanthemyl alcohol
Catalog No.:BCN9908
CAS No.:5617-92-5
- 6-Hydroxy-7-methoxydihydroligustilide
Catalog No.:BCN0168
CAS No.:210036-09-2
- Perlolyrine
Catalog No.:BCN0169
CAS No.:29700-20-7
Inhibited histone deacetylase 3 ameliorates myocardial ischemia-reperfusion injury in a rat model by elevating microRNA-19a-3p and reducing cyclin-dependent kinase 2.[Pubmed:33217223]
IUBMB Life. 2020 Dec;72(12):2696-2709.
OBJECTIVE: Over the years, the roles of microRNAs (miRNAs) and histone deacetylase 3 (HDAC3) in human diseases have been investigated. This study focused on the effect of miR-19a-3p and HDAC3 in myocardial ischemia-reperfusion (I/R) injury (MIRI) by targeting cyclin-dependent kinase 2 (CDK2). METHODS: The I/R rat models were established by coronary artery ligation, which were then treated with RGFP966 (an inhibitor of HDAC3), miR-19a-3p agomir or antagomir, or silenced CDK2 to explore their roles in the cardiac function, pathological changes of myocardial tissues, myocardial infarction area, inflammatory factors and oxidative stress factors in rats with MIRI. The expression of miR-19a-3p, HDAC3, and CDK2 was determined by RT-qPCR and western blot assay, and the interaction among which was also verified by online prediction, luciferase activity assay and ChIP assay. RESULTS: The results indicated that HDAC3 and CDK2 were upregulated while miR-19a-3p was downregulated in myocardial tissues of I/R rats. The inhibited HDAC3/CDK2 or elevated miR-19a-3p could promote cardiac function, attenuate pathological changes, inflammatory reaction, oxidative stress, myocardial infarction area and apoptosis of myocardial tissues. HDAC3 mediates miR-19a-3p and CDK2 is targeted by miR-19a-3p. CONCLUSION: Inhibited HDAC3 ameliorates MIRI in a rat model by elevating miR-19a-3p and reducing CDK2, which may contribute to the treatment of MIRI.
A cell-based bioluminescence assay reveals dose-dependent and contextual repression of AP-1-driven gene expression by BACH2.[Pubmed:33144667]
Sci Rep. 2020 Nov 3;10(1):18902.
Whereas effector CD4(+) and CD8(+) T cells promote immune activation and can drive clearance of infections and cancer, CD4(+) regulatory T (Treg) cells suppress their function, contributing to both immune homeostasis and cancer immunosuppression. The transcription factor BACH2 functions as a pervasive regulator of T cell differentiation, promoting development of CD4(+) Treg cells and suppressing the effector functions of multiple effector T cell (Teff) lineages. Here, we report the development of a stable cell-based bioluminescence assay of the transcription factor activity of BACH2. Tetracycline-inducible BACH2 expression resulted in suppression of phorbol 12-myristate 13-acetate (PMA)/ionomycin-driven activation of a luciferase reporter containing BACH2/AP-1 target sequences from the mouse Ifng + 18k enhancer. BACH2 expression repressed the luciferase signal in a dose-dependent manner but this activity was abolished at high levels of AP-1 signalling, suggesting contextual regulation of AP-1 driven gene expression by BACH2. Finally, using the reporter assay developed, we find that the histone deacetylase 3 (HDAC3)-selective inhibitor, RGFP966, inhibits BACH2-mediated repression of signal-driven luciferase expression. In addition to enabling mechanistic studies, this cell-based reporter may enable identification of small molecule agonists or antagonists of BACH2 function for drug development.
Histone deacetylase 3 aberration inhibits Klotho transcription and promotes renal fibrosis.[Pubmed:33024274]
Cell Death Differ. 2020 Oct 6. pii: 10.1038/s41418-020-00631-9.
Development of renal fibrosis is a hallmark of renal aging and chronic kidney disease of all etiologies and characterized by extensive renal cell injuries and subsequent myofibroblast transdifferentiations (MTDs), which are significantly influenced by aberrant histone deacetylase (HDAC) activities. However, the key HDAC isoforms and effectors that are causally involved in the processes remain poorly understood. Here, we report that aberrant HDAC3 induction and its inhibition of Klotho, a renal epithelium-enriched aging suppressor, contribute significantly to renal fibrogenesis. HDAC3 was preferentially elevated with concomitant Klotho suppression in fibrotic kidneys incurred by unilateral ureter obstruction (UUO) and aristolochic acid nephropathy (AAN), whereas Hdac3 knockout resisted the fibrotic pathologies. The HDAC3 elevation is substantially blocked by the inhibitors of TGFbeta receptor and Smad3 phosphorylation, suggesting that TGFbeta/Smad signal activates Hdac3 transcription. Consistently, an HDAC3-selective inhibitor RGFP966 derepressed Klotho and mitigated the renal fibrotic injuries in both UUO and AAN mice. Further, HDAC3 overexpression or inhibition in renal epithelia inversely affected Klotho abundances and HDAC3 was inducibly associated with transcription regulators NCoR and NF-kB and bound to Klotho promoter in fibrotic kidney, supporting that aberrant HDAC3 targets and transcriptionally inhibits Klotho under renal fibrotic conditions. More importantly, the antirenal fibrosis effects of RGFP966 were largely compromised in mice with siRNA-mediated Klotho knockdown. Hence, HDAC3 aberration and the subsequent Klotho suppression constitute an important regulatory loop that promotes MTD and renal fibrosis and uses of HDAC3-selective inhibitors are potentially effective in treating renal fibrotic disorders.
A Combination of BRD4 and HDAC3 Inhibitors Synergistically Suppresses Glioma Stem Cell Growth by Blocking GLI1/IL6/STAT3 Signaling Axis.[Pubmed:32999044]
Mol Cancer Ther. 2020 Dec;19(12):2542-2553.
Glioma stem cells (GSC) are essential for tumor maintenance, invasiveness, and recurrence. Using a global epigenetic screening with an shRNA library, we identified HDAC3 as an essential factor for GSC stemness. Here, we demonstrated that GSCs poorly respond to an HDAC3 inhibitor, RGFP966 (HDAC3i), owing to the production of IL6 and STAT3 activation. To enhance GSC sensitivity to HDAC3i, we explored whether cotreatment with a BRD4 inhibitor, JQ1 (BRD4i), in GSCs produced a better antitumor effect. BRD4i synergistically inhibits GSC growth in association with HDAC3i. HDAC3 inhibition upregulated the acetylation of H3K27, which allowed the recruitment of BRD4 to the GLI1 gene promoter and induced its expression. GLI1, a transcription factor, turned on the expression of IL6, which led to the activation of STAT3 signaling pathways. However, BRD4i inhibited transcription of the GLI1 gene, thereby blocking the GLI1/IL6/STAT3 pathway. In vivo, the HDAC3i/BRD4i combination caused stronger tumor growth suppression than either drug alone. Thus, HDAC3i/BRD4i might provide promising therapies for GBM.
Targeting HDAC3 in the DBA/2J spontaneous mouse model of glaucoma.[Pubmed:32971093]
Exp Eye Res. 2020 Nov;200:108244.
High intraocular pressure (IOP) is the most common risk factor associated with glaucoma in humans. While lowering IOP is effective at reducing the rate of retinal ganglion cell (RGC) loss, to date, no treatment exists to directly preserve these cells affected by damage to the optic nerve. Recently, histone deacetylase-3 (HDAC3) has become a potential therapeutic target because it plays an important role in the early nuclear atrophic events that precede RGC death. Conditional knockout or inhibition of HDAC3 prevents histone deacetylation, heterochromatin formation, apoptosis, and eventual RGC loss following acute optic nerve injury. Using these approaches to repress HDAC3 activity, we tested whether targeting HDAC3 protects RGCs from ganglion cell-specific BRN3A expression loss, total somatic cell loss, and optic nerve degeneration in the DBA/2J mouse model of spontaneous glaucoma. Targeted ablation of Hdac3 activity did not protect RGCs from axonal degeneration or somatic cell death in the DBA/2J mouse model of glaucoma. However, inhibition of HDAC3 activity using RGFP966 conferred mild protection against somatic cell loss in the ganglion cell layer in aged DBA/2J mice. Further experimentation is necessary to determine whether other class I HDACs may serve as potential therapeutic targets in chronic models of glaucoma.
CaMKII exacerbates heart failure progression by activating class I HDACs.[Pubmed:32971072]
J Mol Cell Cardiol. 2020 Sep 22;149:73-81.
BACKGROUND: Persistent cardiac Ca(2+)/calmodulin dependent Kinase II (CaMKII) activation plays an essential role in heart failure development. However, the molecular mechanisms underlying CaMKII induced heart failure progression remains incompletely understood. Histone deacetylases (HDACs) are critical for transcriptional responses to stress, and contribute to expression of pathological genes causing adverse ventricular remodeling. Class I HDACs, including HDAC1, HDAC2 and HDAC3, promote pathological cardiac hypertrophy, whereas class IIa HDACs suppress cardiac hypertrophy. While it is known that CaMKII deactivates class IIa HDACs to enhance cardiac hypertrophy, the role of CaMKII in regulating class I HDACs during heart failure progression is unclear. METHODS AND RESULTS: CaMKII increases the deacetylase activity of recombinant HDAC1, HDAC2 and HDAC3 via in vitro phosphorylation assays. Phosphorylation sites on HDAC1 and HDAC3 are identified with mass spectrometry. HDAC1 activity is also increased in cardiac-specific CaMKIIdeltaC transgenic mice (CaMKIIdeltaC-tg). Beyond post-translational modifications, CaMKII induces HDAC1 and HDAC3 expression. HDAC1 and HDAC3 expression are significantly increased in CaMKIIdeltaC-tg mice. Inhibition of CaMKII by overexpression of the inhibitory peptide AC3-I in the heart attenuates the upregulation of HDAC1 after myocardial infarction surgery. Importantly, a potent HDAC1 inhibitor Quisinostat improves downregulated autophagy genes and cardiac dysfunction in CaMKIIdeltaC-tg mice. In addition to Quisinostat, selective class I HDACs inhibitors, Apicidin and Entinostat, HDAC3 specific inhibitor RGFP966, as well as HDAC1 and HDAC3 siRNA prevent CaMKII overexpression induced cardiac myocyte hypertrophy. CONCLUSION: CaMKII activates class I HDACs in heart failure, which may be a central mechanism for heart failure progression. Selective class I HDACs inhibition may be a novel therapeutic avenue to alleviate CaMKII hyperactivity induced cardiac dysfunction.
HDAC3 deacetylates the DNA mismatch repair factor MutSbeta to stimulate triplet repeat expansions.[Pubmed:32900932]
Proc Natl Acad Sci U S A. 2020 Sep 22;117(38):23597-23605.
Trinucleotide repeat (TNR) expansions cause nearly 20 severe human neurological diseases which are currently untreatable. For some of these diseases, ongoing somatic expansions accelerate disease progression and may influence age of onset. This new knowledge emphasizes the importance of understanding the protein factors that drive expansions. Recent genetic evidence indicates that the mismatch repair factor MutSbeta (Msh2-Msh3 complex) and the histone deacetylase HDAC3 function in the same pathway to drive triplet repeat expansions. Here we tested the hypothesis that HDAC3 deacetylates MutSbeta and thereby activates it to drive expansions. The HDAC3-selective inhibitor RGFP966 was used to examine its biological and biochemical consequences in human tissue culture cells. HDAC3 inhibition efficiently suppresses repeat expansion without impeding canonical mismatch repair activity. Five key lysine residues in Msh3 are direct targets of HDAC3 deacetylation. In cells expressing Msh3 in which these lysine residues are mutated to arginine, the inhibitory effect of RGFP966 on expansions is largely bypassed, consistent with the direct deacetylation hypothesis. RGFP966 treatment does not alter MutSbeta subunit abundance or complex formation but does partially control its subcellular localization. Deacetylation sites in Msh3 overlap a nuclear localization signal, and we show that localization of MutSbeta is partially dependent on HDAC3 activity. Together, these results indicate that MutSbeta is a key target of HDAC3 deacetylation and provide insights into an innovative regulatory mechanism for triplet repeat expansions. The results suggest expansion activity may be druggable and support HDAC3-selective inhibition as an attractive therapy in some triplet repeat expansion diseases.
RGFP966 inactivation of the YAP pathway attenuates cardiac dysfunction induced by prolonged hypothermic preservation.[Pubmed:32893527]
J Zhejiang Univ Sci B. 2020 Sept.;21(9):703-715.
Oxidative stress and apoptosis are the key factors that limit the hypothermic preservation time of donor hearts to within 4-6 h. The aim of this study was to investigate whether the histone deacetylase 3 (HDAC3) inhibitor RGFP966 could protect against cardiac injury induced by prolonged hypothermic preservation. Rat hearts were hypothermically preserved in Celsior solution with or without RGFP966 for 12 h followed by 60 min of reperfusion. Hemodynamic parameters during reperfusion were evaluated. The expression and phosphorylation levels of mammalian STE20-like kinase-1 (Mst1) and Yes-associated protein (YAP) were determined by western blotting. Cell apoptosis was measured by the terminal deoxynucleotidyl-transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) method. Addition of RGFP966 in Celsior solution significantly inhibited cardiac dysfunction induced by hypothermic preservation. RGFP966 inhibited the hypothermic preservation-induced increase of the phosphorylated (p)-Mst1/Mst1 and p-YAP/YAP ratios, prevented a reduction in total YAP protein expression, and increased the nuclear YAP protein level. Verteporfin (VP), a small molecular inhibitor of YAP-transcriptional enhanced associate domain (TEAD) interaction, partially abolished the protective effect of RGFP966 on cardiac function, and reduced lactate dehydrogenase activity and malondialdehyde content. RGFP966 increased superoxide dismutase, catalase, and glutathione peroxidase gene and protein expression, which was abolished by VP. RGFP966 inhibited hypothermic preservation-induced overexpression of B-cell lymphoma protein 2 (Bcl-2)-associated X (Bax) and cleaved caspase-3, increased Bcl-2 mRNA and protein expression, and reduced cardiomyocyte apoptosis. The antioxidant and anti-apoptotic effects of RGFP966 were cancelled by VP. The results suggest that supplementation of Celsior solution with RGFP966 attenuated prolonged hypothermic preservation-induced cardiac dysfunction. The mechanism may involve inhibition of oxidative stress and apoptosis via inactivation of the YAP pathway.
Histone deacetylase 3 in hippocampus contributes to memory impairment after chronic constriction injury of sciatic nerve in mice.[Pubmed:32868749]
Pain. 2020 Aug 27.
Chronic neuropathic pain is frequently accompanied by memory impairment, yet the underlying mechanisms remain unclear. Here, we showed that mice displayed memory impairment starting at 14 days and lasting for at least 21 days after chronic constriction injury (CCI) of unilateral sciatic nerve in mice. Systemic administration of the pan histone deacetylase (HDAC) inhibitor sodium butyrate attenuated this memory impairment. More specifically, we found that hippocampus HDAC3 was involved in this process because the levels of its mRNA and protein increased significantly in the hippocampus at 14 and 21 days after CCI, but not sham surgery. Systemic administration of the selective HDAC3 antagonist RGFP966 attenuated CCI-induced memory impairment, improved hippocampal long-term potentiation impairment, and rescued reductions of dendritic spine density and synaptic plasticity-associated protein in the hippocampus. In addition, HDAC3 overexpression in the hippocampus led to memory impairment without affecting basal nociceptive responses in naive mice. Our findings suggest that HDAC3 contributes to memory impairment after CCI by impairing synaptic plasticity in hippocampus. Histone deacetylase 3 might serve as a potential molecular target for therapeutic treatment of memory impairment under neuropathic pain conditions.
Histone deacetylase 3-selective inhibitor RGFP966 ameliorates impaired glucose tolerance through beta-cell protection.[Pubmed:32800772]
Toxicol Appl Pharmacol. 2020 Nov 1;406:115189.
The potential therapeutic effect of histone deacetylase 3 (HDAC3) pharmacologic inhibition on diabetes has been focused recently. RGFP966, as a highly-selective HDAC3 inhibitor, its possible roles and underlying mechanism in the treatment of diabetes needs to be clarified. In this study, low-dose streptozotocin (STZ)-induced pre-diabetic mice were used to test the regulatory ability of RGFP966 in blood glucose and insulin. We isolated the islets both from normal C57BL/6 J mice and KKA(y) mice with spontaneous type 2 diabetes to determine the potency of RGFP966 on glucose-stimulated insulin secretion. NIT-1 pancreatic beta-cells induced by sodium palmitate (PA) were applied to identify the protective effects of RGFP966 against beta-cell apoptosis. The results showed that administration of RGFP966 in the pre-diabetic mice not only significantly reduced hyperglycemia, promoted phase I insulin secretion, improved morphology of islets, but also increased glucose infusion rate (GIR) during hyperglycemic clamp test. When treated in vitro, RGFP966 enhanced insulin secretion and synthesis in islets of normal C57BL/6J mice and diabetic KKA(y) mice. In addition, it partially attenuated PA-induced apoptosis in NIT-1 cells. Therefore, our research suggests that RGFP966, probably through selective inhibition of HDAC3, might serve as a novel potential preventive and therapeutic candidate for diabetes.
RGFP966, a histone deacetylase 3 inhibitor, promotes glioma stem cell differentiation by blocking TGF-beta signaling via SMAD7.[Pubmed:32585142]
Biochem Pharmacol. 2020 Oct;180:114118.
Glioma stem cells (GSC) play a major role in drug resistance and tumor recurrence. Using a genetic screen with a set of shRNAs that can target chromatin regulators in a GSC model, we have HDAC3 as a major negative regulator of GSC differentiation. Inhibition of HDAC3 using a pharmacological inhibitor or a siRNA led to the induction of GSC differentiation into astrocytes. Consequently, HDAC3-inhibition also caused a strong reduction of tumor-promoting and self-renewal capabilities of GSCs. These phenotypes were highly associated with an increased acetylation of SMAD7, which protected its ubiquitination. SMAD7 inhibits a TGF-beta signaling axis that is required for maintaining stemness. These results demonstrate that HDAC3 appears to be a proper target in anti-glioma therapy.
Inactivation of BRM/SMARCA2 sensitizes clear cell renal cell carcinoma to histone deacetylase complex inhibitors.[Pubmed:32067803]
Pathol Res Pract. 2020 Apr;216(4):152867.
BRM, a key subunit of the SWI/SNF chromatin remodeling complex, is an important tumor suppressor gene in multiple tumors. BRM is not mutated, but rather epigenetically silenced in a variety of tumor types, which is different from many anti-cancer genes. In addition, histone deacetylase complex (HDAC) inhibitors are known to reverse BRM silencing, but they also inactivate it via acetylation of its c-terminus. HDAC inhibitors have been reported to be effective at pharmacologically restoring BRM and thereby inhibiting cancer cell growth. But we do not know which HDAC inhibitor, if any, regulate BRM in clear cell renal cell carcinoma (RCC). By using seven types of HDAC inhibitors, we found that Pan-HDAC inhibitors restored BRM protein expression. Despite their ability to restore BRM expression, these HDAC inhibitors also blocked BRM function when present. However, after their removal, we observed that BRM expression remained elevated for several days, and during this period, BRM activity was detected. In addition, HDAC3 and HDAC9 regulate BRM expression and function, especially for HDAC3 inhibitor, RGFP966. Our study demonstrated that knockdown of BRM promoted RCC cells proliferation, migration and invasion. RGFP966 inhibited the tumor progression of clear cell RCC by restoring BRM expression both in vivo and in vitro. In conclusion, HDAC3 is potential targets for clinical treatment, and our study provides a new approach for targeted therapy of BRM-negative clear cell RCC.
RGFP966 Suppresses Tumor Growth and Migration Through Inhibition of EGFR Expression in Hepatocellular Carcinoma Cells in vitro.[Pubmed:32021097]
Drug Des Devel Ther. 2020 Jan 10;14:121-128.
Purpose: Histone deacetylase 3 (HDAC3) has been suggested to play a role in hepatocellular carcinoma (HCC). In the present report, we aimed to identify the effects of RGFP966, a specific HDAC3 inhibitor, on the cell proliferation and migration of HCC cell lines. Methods: Human HCC cell lines, which were identified using short tandem repeat (STR) DNA profiling analysis, were used in this report. Cell proliferation assay was used to identify the growth viability of cells. Wound healing and transwell assay were used to identify the migration ability of cells. Further, a human phospho-receptor tyrosine kinases array kit was used to screen out RGFP966 effects on key receptor tyrosine kinases. Then, the mRNA expression was quantified by real-time PCR, and protein expression was identified by Western blot immunoassay. Results: We found that RGFP966 inhibited both proliferation and migration of HCC cells. Further, RGFP966 represses the expression and phosphorylation levels of epidermal growth factor receptor (EGFR) in HCC cells. Moreover, HDAC3 is involved in the inhibition of EGFR by RGFP966. Overall, we elucidated an inhibitive function of RGFP966 in HCC progression. Conclusion: RGFP966 inhibits EGFR signaling pathway and suppresses proliferation and migration of HCC cells.
Neuroprotective Effects of Selective Inhibition of Histone Deacetylase 3 in Experimental Stroke.[Pubmed:32016769]
Transl Stroke Res. 2020 Oct;11(5):1052-1063.
Histone deacetylase 3 (HDAC3) has been implicated as neurotoxic in several neurodegenerative conditions. However, the role of HDAC3 in ischemic stroke has not been thoroughly explored. We tested the hypothesis that selective inhibition of HDAC3 after stroke affords neuroprotection. Adult male Wistar rats (n = 8/group) were subjected to 2 h of middle cerebral artery occlusion (MCAO), and randomly selected animals were treated intraperitoneally twice with either vehicle (1% Tween 80) or a selective HDAC3 inhibitor (RGFP966, 10 mg/kg) at 2 and 24 h after MCAO. Long-term behavioral tests were performed up to 28 days after MCAO. Another set of rats (n = 7/group) were sacrificed at 3 days for histological analysis. Immunostaining for HDAC3, acetyl-Histone 3 (AcH3), NeuN, TNF-alpha, toll-like receptor 4 (TLR4), cleaved caspase-3, cleaved poly (ADP-ribose) polymerase (PARP), Akt, and TUNEL were performed. Selective HDAC3 inhibition improved long-term functional outcome (p < 0.05) and reduced infarct volume (p < 0.0001). HDAC3 inhibition increased levels of AcH3 in the ischemic brain (p = 0.016). Higher levels of AcH3 were significantly correlated with better neurological scores and smaller infarct volumes (r = 0.74, p = 0.002; r = 0.6, p = 0.02, respectively). The RGFP966 treatment reduced apoptosis-TUNEL+, cleaved caspase-3+, and cleaved PARP+ cells-and neuroinflammation-TNF-alpha+ and TLR4+ cells-in the ischemic border compared to vehicle control (p < 0.05). The RGFP966 treatment also increased Akt expression in the ipsilateral cortex (p < 0.001). Selective HDAC3 inhibition after stroke improves long-term neurological outcome and decreases infarct volume. The neuroprotective effects of HDAC3 inhibition are associated with a reduction in apoptosis and inflammation and upregulation of the Akt pathway.


