MaritimeinCAS# 490-54-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

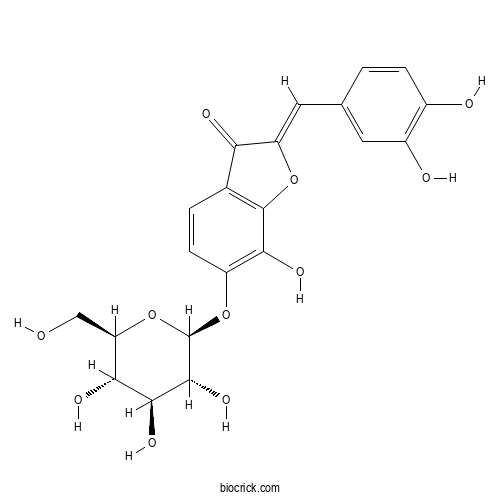
| Cas No. | 490-54-0 | SDF | Download SDF |
| PubChem ID | 6450184 | Appearance | Powder |
| Formula | C21H20O11 | M.Wt | 448.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2Z)-2-[(3,4-dihydroxyphenyl)methylidene]-7-hydroxy-6-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-1-benzofuran-3-one | ||
| SMILES | C1=CC(=C(C=C1C=C2C(=O)C3=C(O2)C(=C(C=C3)OC4C(C(C(C(O4)CO)O)O)O)O)O)O | ||
| Standard InChIKey | SYRURBPRFQUYQS-RHEJLWEFSA-N | ||
| Standard InChI | InChI=1S/C21H20O11/c22-7-14-16(26)18(28)19(29)21(32-14)31-12-4-2-9-15(25)13(30-20(9)17(12)27)6-8-1-3-10(23)11(24)5-8/h1-6,14,16,18-19,21-24,26-29H,7H2/b13-6-/t14-,16-,18+,19-,21-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reference standards. | |||||

Maritimein Dilution Calculator

Maritimein Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2302 mL | 11.1508 mL | 22.3015 mL | 44.603 mL | 55.7538 mL |
| 5 mM | 0.446 mL | 2.2302 mL | 4.4603 mL | 8.9206 mL | 11.1508 mL |
| 10 mM | 0.223 mL | 1.1151 mL | 2.2302 mL | 4.4603 mL | 5.5754 mL |
| 50 mM | 0.0446 mL | 0.223 mL | 0.446 mL | 0.8921 mL | 1.1151 mL |
| 100 mM | 0.0223 mL | 0.1115 mL | 0.223 mL | 0.446 mL | 0.5575 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3',4',7-Trihydroxyisoflavone
Catalog No.:BCN0149
CAS No.:485-63-2
- 2'-Hydroxyflavanone
Catalog No.:BCN0148
CAS No.:17348-76-4
- 2',6'-Dihydroxy 4',4-dimethoxydihydrochalcone
Catalog No.:BCN0147
CAS No.:35241-54-4
- Daphnin
Catalog No.:BCN0146
CAS No.:486-55-5
- 3',4',7,8-Tetrahydroxyflavone
Catalog No.:BCN0145
CAS No.:3440-24-2
- Ergocristine
Catalog No.:BCN0144
CAS No.:511-08-0
- cis-Aconitic acid
Catalog No.:BCN0143
CAS No.:585-84-2
- alpha-Bisabolol
Catalog No.:BCN0142
CAS No.:23089-26-1
- 7-Hydroxyflavonol
Catalog No.:BCN0141
CAS No.:492-00-2
- (+/-)-2-Methyl-1-butanol
Catalog No.:BCN0140
CAS No.:137-32-6
- Syringetin 3-O-galactoside
Catalog No.:BCN0139
CAS No.:55025-56-4
- Cynatratoside E
Catalog No.:BCN0138
CAS No.:
- Candicine
Catalog No.:BCN0151
CAS No.:6656-13-9
- Comanthosid B
Catalog No.:BCN0153
CAS No.:70938-60-2
- Helenien
Catalog No.:BCN0154
CAS No.:547-17-1
- N-trans-caffeoyltyramine
Catalog No.:BCN0155
CAS No.:103188-48-3
- Lappaol B
Catalog No.:BCN0156
CAS No.:62359-60-8
- 2-Hydroxy-3-methoxybenzaldehyde
Catalog No.:BCN0157
CAS No.:148-53-8
- (E)-N-(2-Amino-4-fluorophenyl)-3-(1-cinnamyl-1H-pyrazol-4-yl)acrylamide
Catalog No.:BCN0158
CAS No.:1396841-57-8
- Bicyclo[5.3.0]decapentaene
Catalog No.:BCN0159
CAS No.:275-51-4
- 2,5-Dimethoxybenzoic acid
Catalog No.:BCN0160
CAS No.:2785-98-0
- 2,3-Dimethylanthraquinone
Catalog No.:BCN0161
CAS No.:6531-35-7
- Qingdainone
Catalog No.:BCN0162
CAS No.:97457-31-3
- 7-Hydroxy-4'-methoxyflavone
Catalog No.:BCN0163
CAS No.:487-24-1
Comparison of antioxidant activities of different parts from snow chrysanthemum (Coreopsis tinctoria Nutt.) and identification of their natural antioxidants using high performance liquid chromatography coupled with diode array detection and mass spectrometry and 2,2'-azinobis(3-ethylbenzthiazoline-sulfonic acid)diammonium salt-based assay.[Pubmed:26521095]
J Chromatogr A. 2016 Jan 8;1428:134-42.
Snow chrysanthemum (Coreopsis tinctoria Nutt.), a world-widely well-known flower tea material, has attracted more and more attention because of its beneficial health effects such as antioxidant activity and special flavor. In this study, a high performance liquid chromatography coupled with diode array detection and mass spectrometry (HPLC-DAD-MS) and 2,2'-azinobis(3-ethylbenzthiazoline-sulfonic acid)diammonium salt (ABTS) based assay was employed for comparison and identification of antioxidants in different samples of snow chrysanthemum. The results showed that snow chrysanthemum flowers possessed the highest while stems presented the lowest antioxidant capacities. Fourteen detected peaks with antioxidant activity were temporarily identified as 3,4',5,6,7-pentahydroxyflavanone-O-hexoside, chlorogenic acid, 2R-3',4',8-trihydroxyflavanone-7-O-glucoside, flavanomarein, flavanocorepsin, flavanokanin, quercetagitin-7-O-glucoside, 3',5,5',7-tetrahydroxyflavanone-O-hexoside, marein, Maritimein, 1,3-dicaffeoylquinic acid, coreopsin, okanin and acetyl-marein by comparing their UV spectra, retention times and MS data with standards or literature data. Antioxidants existed in snow chrysanthemum are quite different from those reported in Chrysanthemum morifolium, a well-known traditional beverage in China, which indicated that snow chrysanthemum may be a promising herbal tea material with obvious antioxidant activity.
Potential natural sensitizers extracted from the skin of Canarium odontophyllum fruits for dye-sensitized solar cells.[Pubmed:25541396]
Spectrochim Acta A Mol Biomol Spectrosc. 2015 Mar 5;138:596-602.
Possibility of use of dye extract from skin samples of a seasonal, indigenous fruit from Borneo, namely Canarium odontophyllum, in dye sensitized solar cells (DSSCs) are explored. Three main groups of flavonoid pigments are detected and these pigments exhibit different UV-vis absorption properties, and hence showing different light harvesting capabilities. When applied in DSSCs. The detected pigment constituents of the extract consist of aurone (Maritimein), anthocyanidin (pelargonidin) and anthocyanidin (cyanidin derivatives). When tested in DSSC, the highest conversion efficiency of 1.43% is exhibited by cyanidin derivatives, and this is followed by conversion efficiencies of 0.51% and 0.79% for aurone and pelargonidin, respectively. It is shown that individual pigments, like cyanidin derivatives and pelargonidin, exhibit higher power conversion efficiency when compared to that of C.odontophyllum skin pigment mixture (with a conversion efficiency of only 0.68%). The results indicate a possibility of masking effects of the pigments when used as a mixture. The acidification of C.odontophyllum skin pigments with concentrated hydrochloric acid improves the conversion efficiency of the mixture from 0.68% to 0.99%. The discussion in this paper will draw data and observations from the variation in absorption and adsorption properties, the HOMO-LUMO levels, the energy band gaps and the functional group compositions of the detected flavonoids.
[Polyphenolic substances of Cynara scolymus L. leaves].[Pubmed:2610472]
Ann Pharm Fr. 1989;47(2):95-8.
From the leaves of Cynara scolymus the following substances where isolated: apigenin, luteolin, luteolin-4'-glucoside, cynaroside, scolimoside, cosmoside, quercetin, rutin, chlorogenic acid, caffeic acid, isochlorogenic acid, luteolin-7-gentiobioside, along with the more uncommon scopoletin, hesperitin, hesperidoside, esculetin-6-O-beta-glucoside; more over Maritimein was for the first time isolated and identified in the genus.


