HelenienCAS# 547-17-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 547-17-1 | SDF | Download SDF |
| PubChem ID | 5281240 | Appearance | Powder |
| Formula | C72H116O4 | M.Wt | 1045.7 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
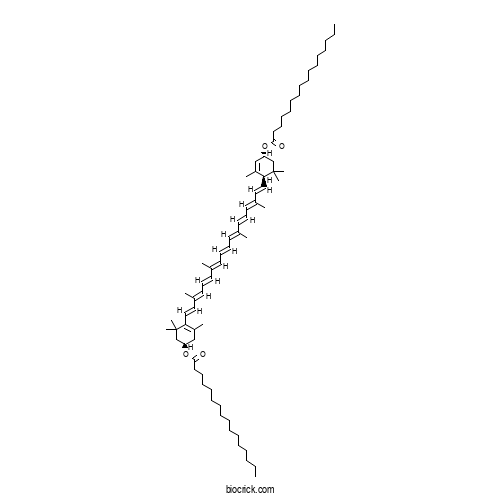
| Chemical Name | [(1R)-4-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(1R,4R)-4-hexadecanoyloxy-2,6,6-trimethylcyclohex-2-en-1-yl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-3,5,5-trimethylcyclohex-3-en-1-yl] hexadecanoate | ||
| SMILES | CCCCCCCCCCCCCCCC(=O)OC1CC(=C(C(C1)(C)C)C=CC(=CC=CC(=CC=CC=C(C)C=CC=C(C)C=CC2C(=CC(CC2(C)C)OC(=O)CCCCCCCCCCCCCCC)C)C)C)C | ||
| Standard InChIKey | YHGJHDJZIOYZIR-URPSFYETSA-N | ||
| Standard InChI | InChI=1S/C72H116O4/c1-13-15-17-19-21-23-25-27-29-31-33-35-37-49-69(73)75-65-55-63(7)67(71(9,10)57-65)53-51-61(5)47-41-45-59(3)43-39-40-44-60(4)46-42-48-62(6)52-54-68-64(8)56-66(58-72(68,11)12)76-70(74)50-38-36-34-32-30-28-26-24-22-20-18-16-14-2/h39-48,51-55,65-67H,13-38,49-50,56-58H2,1-12H3/b40-39+,45-41+,46-42+,53-51+,54-52+,59-43+,60-44+,61-47+,62-48+/t65-,66+,67-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reference standards. | |||||

Helenien Dilution Calculator

Helenien Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.9563 mL | 4.7815 mL | 9.563 mL | 19.1259 mL | 23.9074 mL |
| 5 mM | 0.1913 mL | 0.9563 mL | 1.9126 mL | 3.8252 mL | 4.7815 mL |
| 10 mM | 0.0956 mL | 0.4781 mL | 0.9563 mL | 1.9126 mL | 2.3907 mL |
| 50 mM | 0.0191 mL | 0.0956 mL | 0.1913 mL | 0.3825 mL | 0.4781 mL |
| 100 mM | 0.0096 mL | 0.0478 mL | 0.0956 mL | 0.1913 mL | 0.2391 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Comanthosid B
Catalog No.:BCN0153
CAS No.:70938-60-2
- Candicine
Catalog No.:BCN0151
CAS No.:6656-13-9
- Maritimein
Catalog No.:BCN0150
CAS No.:490-54-0
- 3',4',7-Trihydroxyisoflavone
Catalog No.:BCN0149
CAS No.:485-63-2
- 2'-Hydroxyflavanone
Catalog No.:BCN0148
CAS No.:17348-76-4
- 2',6'-Dihydroxy 4',4-dimethoxydihydrochalcone
Catalog No.:BCN0147
CAS No.:35241-54-4
- Daphnin
Catalog No.:BCN0146
CAS No.:486-55-5
- 3',4',7,8-Tetrahydroxyflavone
Catalog No.:BCN0145
CAS No.:3440-24-2
- Ergocristine
Catalog No.:BCN0144
CAS No.:511-08-0
- cis-Aconitic acid
Catalog No.:BCN0143
CAS No.:585-84-2
- alpha-Bisabolol
Catalog No.:BCN0142
CAS No.:23089-26-1
- 7-Hydroxyflavonol
Catalog No.:BCN0141
CAS No.:492-00-2
- N-trans-caffeoyltyramine
Catalog No.:BCN0155
CAS No.:103188-48-3
- Lappaol B
Catalog No.:BCN0156
CAS No.:62359-60-8
- 2-Hydroxy-3-methoxybenzaldehyde
Catalog No.:BCN0157
CAS No.:148-53-8
- (E)-N-(2-Amino-4-fluorophenyl)-3-(1-cinnamyl-1H-pyrazol-4-yl)acrylamide
Catalog No.:BCN0158
CAS No.:1396841-57-8
- Bicyclo[5.3.0]decapentaene
Catalog No.:BCN0159
CAS No.:275-51-4
- 2,5-Dimethoxybenzoic acid
Catalog No.:BCN0160
CAS No.:2785-98-0
- 2,3-Dimethylanthraquinone
Catalog No.:BCN0161
CAS No.:6531-35-7
- Qingdainone
Catalog No.:BCN0162
CAS No.:97457-31-3
- 7-Hydroxy-4'-methoxyflavone
Catalog No.:BCN0163
CAS No.:487-24-1
- 5-Hydroxyflavone
Catalog No.:BCN0164
CAS No.:491-78-1
- Yohimbic acid monohydrate
Catalog No.:BCN0165
CAS No.:522-87-2
- Ethyl isovalerate
Catalog No.:BCN0166
CAS No.:108-64-5
Effect of nilvadipine on central visual field in retinitis pigmentosa: a 30-month clinical trial.[Pubmed:20948238]
Ophthalmologica. 2011;225(2):120-6.
PURPOSE: To assess the effects of nilvadipine on the progression of central visual field defect in retinitis pigmentosa (RP). DESIGN: Prospective, randomized, nonmasked, single-center trial. METHODS: Patients with RP were randomly divided into a treated group receiving oral nilvadipine at 4 mg/day for >/=30 months and a control group receiving tocopherol nicotinate at 300 mg/day, Helenien at 15 mg/day or no medication for the same periods. Progression of RP was evaluated using the 10-2 SITA Fast Program of the Humphrey Visual Field Analyzer, and regression coefficients calculated from the time courses of mean deviation (MD slope) were compared between groups. RESULTS: Nineteen patients in the treated group and 14 patients in the control group completed the follow-up for >/=30 months. The mean (+/-standard deviation) duration of observation was 48.8 +/- 11.8 months (median 48 months, range 30-66 months) for the treated group and 49.2 +/- 18.1 months (median 48 months, range 30-90 months) for the control group (p = 0.94). Mean (+/-standard error of the mean, SEM) regression coefficients of the averaged MD values for the initial 30 months were -0.35 +/- 0.17 dB/year in the treated group and -0.75 +/- 0.06 dB/year in the control group (p < 0.01). Mean (+/-SEM) MD slopes for total observational periods were -0.49 +/- 0.17 dB/year in the treated group and -0.89 +/- 0.16 dB/year in the control group (mean +/- SEM, p = 0.042). CONCLUSION: Nilvadipine at 4 mg/day significantly retarded progression of central visual field defects in RP in this small patient series.
[Effects of cyaninoside chloride and Heleniene on mesopic and scotopic vision in myopia and night blindness].[Pubmed:6381579]
J Fr Ophtalmol. 1984;7(1):35-9.
A controlled, clinical trial, comparing cyaninoside chloride and Heleniene , was conducted on 31 out-patients suffering from functional disturbances of vision in low-luminance conditions. The evolution of photopic and mesopic visual acuities, electro- oculograms and adapto -electroretinograms was assessed for both treatment groups and controls. Both agents significantly improved photopic visual acuity (p less than 0.05). Only cyaninoside chloride treatment improved visual functions related to mesopic and scotopic vision (p less than 0.01). There were also significant differences between the two treatment groups regarding the velocity of visual adaptation in adapto -electroretinography. This study thus demonstrates the therapeutic value of cyaninoside chloride for the treatment of functional disturbances of mesopic and scotopic vision, especially in night blindness and myopia.


