CandicineCAS# 6656-13-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

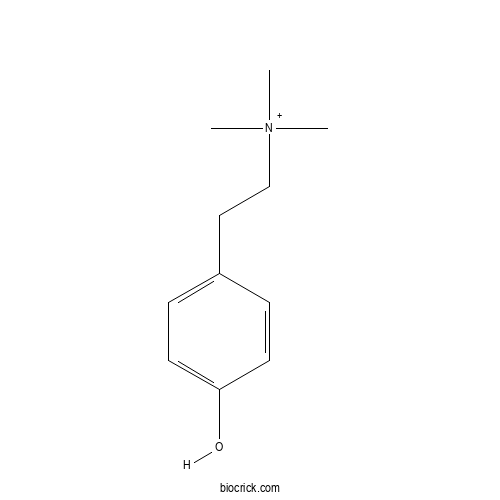
| Cas No. | 6656-13-9 | SDF | Download SDF |
| PubChem ID | 23135 | Appearance | Powder |
| Formula | C11H18NO | M.Wt | 180.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(4-hydroxyphenyl)ethyl-trimethylazanium | ||
| SMILES | C[N+](C)(C)CCC1=CC=C(C=C1)O | ||
| Standard InChIKey | PTOJXIKSKSASRB-UHFFFAOYSA-O | ||
| Standard InChI | InChI=1S/C11H17NO/c1-12(2,3)9-8-10-4-6-11(13)7-5-10/h4-7H,8-9H2,1-3H3/p+1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Candicine Dilution Calculator

Candicine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.5463 mL | 27.7316 mL | 55.4631 mL | 110.9262 mL | 138.6578 mL |
| 5 mM | 1.1093 mL | 5.5463 mL | 11.0926 mL | 22.1852 mL | 27.7316 mL |
| 10 mM | 0.5546 mL | 2.7732 mL | 5.5463 mL | 11.0926 mL | 13.8658 mL |
| 50 mM | 0.1109 mL | 0.5546 mL | 1.1093 mL | 2.2185 mL | 2.7732 mL |
| 100 mM | 0.0555 mL | 0.2773 mL | 0.5546 mL | 1.1093 mL | 1.3866 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Maritimein
Catalog No.:BCN0150
CAS No.:490-54-0
- 3',4',7-Trihydroxyisoflavone
Catalog No.:BCN0149
CAS No.:485-63-2
- 2'-Hydroxyflavanone
Catalog No.:BCN0148
CAS No.:17348-76-4
- 2',6'-Dihydroxy 4',4-dimethoxydihydrochalcone
Catalog No.:BCN0147
CAS No.:35241-54-4
- Daphnin
Catalog No.:BCN0146
CAS No.:486-55-5
- 3',4',7,8-Tetrahydroxyflavone
Catalog No.:BCN0145
CAS No.:3440-24-2
- Ergocristine
Catalog No.:BCN0144
CAS No.:511-08-0
- cis-Aconitic acid
Catalog No.:BCN0143
CAS No.:585-84-2
- alpha-Bisabolol
Catalog No.:BCN0142
CAS No.:23089-26-1
- 7-Hydroxyflavonol
Catalog No.:BCN0141
CAS No.:492-00-2
- (+/-)-2-Methyl-1-butanol
Catalog No.:BCN0140
CAS No.:137-32-6
- Syringetin 3-O-galactoside
Catalog No.:BCN0139
CAS No.:55025-56-4
- Comanthosid B
Catalog No.:BCN0153
CAS No.:70938-60-2
- Helenien
Catalog No.:BCN0154
CAS No.:547-17-1
- N-trans-caffeoyltyramine
Catalog No.:BCN0155
CAS No.:103188-48-3
- Lappaol B
Catalog No.:BCN0156
CAS No.:62359-60-8
- 2-Hydroxy-3-methoxybenzaldehyde
Catalog No.:BCN0157
CAS No.:148-53-8
- (E)-N-(2-Amino-4-fluorophenyl)-3-(1-cinnamyl-1H-pyrazol-4-yl)acrylamide
Catalog No.:BCN0158
CAS No.:1396841-57-8
- Bicyclo[5.3.0]decapentaene
Catalog No.:BCN0159
CAS No.:275-51-4
- 2,5-Dimethoxybenzoic acid
Catalog No.:BCN0160
CAS No.:2785-98-0
- 2,3-Dimethylanthraquinone
Catalog No.:BCN0161
CAS No.:6531-35-7
- Qingdainone
Catalog No.:BCN0162
CAS No.:97457-31-3
- 7-Hydroxy-4'-methoxyflavone
Catalog No.:BCN0163
CAS No.:487-24-1
- 5-Hydroxyflavone
Catalog No.:BCN0164
CAS No.:491-78-1
[Comparison of chemical constituents of wild silkworm cocoon and domestic silkworm cocoon by UHPLC-MS technology].[Pubmed:31441626]
Sheng Wu Gong Cheng Xue Bao. 2019 Aug 25;35(8):1546-1556.
Identifying and comparing the chemical constituents of wild silkworm cocoon and silkworm cocoon is of great significance for understanding the domestication of silkworm. In this study, we used high temperature and high pressure and methanol-water system to extract cocoon chemical constituents. We used UHPLC-MS to identify and compare cocoon chemical constituents of wild silkworm and domestic silkworm Dazao and Haoyue strains. The cocoon metabolic fingerprints of wild silkworm and domestic silkworm Dazao and Haoyue strains were obtained by using the UHPLC-MS in the positive ion mode and negative ion mode. By annotation, we found that cocoon chemical compounds with high abundances contained amino acids, flavonoids, alkaloids, terpenes, organic acids, and lignans. PLS-DA showed that the cocoon components were significantly different among the wild silkworm and two domestic silkworm strains Dazao and Haoyue. Proline, leucine/isoleucine and phenylalanine showed significantly higher abundances in the cocoon of domestic silkworm Dazao strain than in those of wild silkworm and domestic silkworm Haoyue strain. The flavonoid secondary metabolites are abundant in the Dazao cocoon, including quercetin, isoquercetin, quercetin 3-O-sophoroside, quercetin-3-O-alpha-L-rhamnoside, quercetin-3-O- rutinoside, and kaempferol. The other secondary metabolites, alkaloids, terpenes and lignans, showed higher abundances in the wild silkworm cocoon than in the domestic silkworm cocoon, including neurine, Candicine, pilocarpidine, artemisiifolin, eupassopin, and eudesobovatol. By exposing cocoons to UV light and observing the green fluorescence of flavonoids, we found that Dazao cocoon had the most flavonoids, and Haoyue cocoon had least flavonoids and wild silkworm cocoon had mediate flavonoids. Alkaloids and organic acids are good anti-insect and antimicrobial agents, which have high abundance in the wild silkworm cocoon and could enhance the defense ability of wild silkworm cocoon. Flavonoids are abundant in the cocoon of domestic silkworm Dazao strain, which the main factors are leading to the yellow-green cocoon of Dazao.
Blood-brain barrier specific permeability assay reveals N-methylated tyramine derivatives in standardised leaf extracts and herbal products of Ginkgo biloba.[Pubmed:27592255]
J Pharm Biomed Anal. 2016 Nov 30;131:167-174.
The linkage between the central nervous system availability and neuropharmacological activity of the constituents of Ginkgo biloba L. extracts (GBE) is still incomplete. In this study, the in vitro blood-brain barrier (BBB) permeability profile of the standardised GBE was investigated by the parallel artificial membrane permeability assay (PAMPA). Biomarkers, such as terpene trilactones, flavonoid aglycones and ginkgotoxin exerted moderate or good BBB-permeability potential (BBB+), while glycosides and biflavones were predicted as unable to pass the BBB. N-methyltyramine (NMT) and N,N-dimethyltyramine or hordenine (Hor) were identified among BBB+ compounds, while subsequent direct HRMS analysis revealed tyramine (Tyr) and N,N,N-trimethyltyramine or Candicine (Can) in GBE as trace constituents. Distribution of Tyr, NMT, Hor and Can was determined by a validated ion-exchange mechanism-based liquid chromatography-electrospray ionisation-mass spectrometry (LC-ESI-MS) method in G. biloba samples, such as herbal drugs and dietary supplements. The total content of the four tyramine derivatives in various GBEs ranged from 7.3 up to 6357mug/g dry extract with NMT and Hor as most abundant ones. Considering the pharmacological activities and the revealed fluctuation in the concentration of the analysed adrenergic protoalkaloids, the presented rapid LC-ESI-MS method is proposed for monitoring of the levels of Tyr, NMT, Hor and Can in G. biloba products.
N-methylated derivatives of tyramine in citrus genus plants: identification of N,N,N-trimethyltyramine (candicine).[Pubmed:24635695]
J Agric Food Chem. 2014 Mar 26;62(12):2679-84.
The distribution of tyramine and its methylated derivatives, N-methyltyramine and N,N-dimethyltyramine, was investigated in tissue parts (leaves and fruits) of several plants of Citrus genus by liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS). In the course of our study we discovered the occurrence of N,N,N-trimethyltyramine in all citrus plants examined. This quaternary ammonium compound, known to act in animals as a neurotoxin, was recognized and characterized by mass spectrometric analysis. The substance, never described before in the Citrus genus, is also known as Candicine or maltoxin. Results indicate that N,N,N-trimethyltyramine is consistently expressed in leaves of clementine, bitter orange, and lemon. Conversely, low levels were found in the leaves of orange, mandarin, chinotto (Citrus myrtifolia), bergamot, citron, and pomelo. In the edible part of the fruits, N,N,N-trimethyltyramine was found at trace levels.
Phenylalkylamine alkaloids from Stapelia hirsuta L.[Pubmed:16753902]
Nat Prod Res. 2006 Jul 10;20(8):710-4.
Four alkaloids of the phenethylamine derivatives have been isolated from the n-butanol fraction of the aerial parts of Stapelia hirsuta L. The structures of the isolated alkaloids were determined as N-acetyl hordenine (a new natural compound), hordenine, Candicine and hordenine-1-O-beta-D-glucoside, in addition to luteolin-7-O-beta-D-glucopyranoside.
Novel quinoline alkaloid from trunk bark of Galipea officinalis.[Pubmed:11449523]
Fitoterapia. 2000 Sep;71(5):605-6.
The isolation of N-methyl-4-hydroxy-3-(2',3'-epoxyisobutyl)-2-quinolone (1), a new natural compound, and Candicine (2) from Galipea officinalis trunk bark is reported. This is the first report of Candicine in the genus Galipea.
Indole-, imidazole- and phenyl-alkylamines in the skin of one hundred and forty American amphibian species other than bufonids.[Pubmed:2877780]
Comp Biochem Physiol C Comp Pharmacol Toxicol. 1986;85(1):139-47.
Extracts prepared from dried or fresh skins of 140 American amphibian species, other than bufonids, were subjected to chemical and biological screening in order to determine the presence and concentrations of aromatic biogenic amines. The most frequent and abundantly occurring amine category was that of indolealkylamines, represented by their prototype 5-hydroxytryptamine and its N-methylated derivatives. Conjugated and cyclized indolealkylamines, typical for the toad skin, were apparently lacking. Phenylalkylamines were represented by two quaternary ammonium bases: leptodactyline and, very rarely, Candicine. Leptodactyline was particularly abundant in leptodactylid frogs of the genus Leptodactylus. Histamine occurred in trace amounts in different species, in large amounts only in some Leptodactylus species of the "pachypus" section. On the other hand, N-methylated histamines and cyclized histamines (spinaceamines) were confined to the skin of Leptodactylus pentadactylus labyrinthicus. The possible taxonomical and evolutionary significance of amphibian skin amines is pointed out.
Candicine from Stapelia gigantea.[Pubmed:17402051]
Planta Med. 1981 Nov;43(11):304-6.
Hordenine (N, N-dimethyltyramine) was found previously in the title plant. The detection of tyramine, N-methyltyramine, and choline and the crystallization of Candicine (N, N, N-trimethyltyramine chloride) is now reported for this species.
Alkaloids as inhibitors of photophosphorylation in spinach chloroplasts.[Pubmed:19397001]
Biochim Biophys Acta. 1974 Jan 18;333(1):141-8.
A group of 12 alkaloids were tested as inhibitors of photophosphorylation in spinach chloroplasts. Ajmaline, a dihydroindole alkaloid, was found to be the strongest inhibitor of both cyclic and non-cyclic photophosphorylation. Low concentrations of ajmaline also inhibited the dark and light ATPases, and the coupled electron flow from water to ferricyanide, measured either as ferrocyanide formed or as oxygen evolved, but not the uncoupled electron transport or the pH rise of illuminated unbuffered suspensions of chloroplasts. Higher concentrations of ajmaline stimulated, instead of inhibiting, photosynthetic electron transport or oxygen evolution and decreased the pH rise, thus behaving as an uncoupler, such as ammonia. Photophosphorylation was partially inhibited by 100 microM dihydrosanguinarine, 100 microM dihydrochelerythrine (benzophenanthridine alkaloids); 500 microM O,O'-dimethylmagnoflorine, 500 microM N-methylcorydine (aporphine alkaloids) and 1 mM julocrotine. They also inhibited coupled oxygen evolution and only partially (dihydrosanguinarine and dihydrochelerythrine) or not at all (the other alkaloids) uncoupled oxygen evolution. Spegazzinine (dihydroindole alkaloid), magnoflorine, N-methylisocorydine, coryneine (aporphine alkaloids), Candicine and ribalinium chloride were without effect on photophosphorylation at 500 microM.


