Procyanidin B3CAS# 23567-23-9 |
- Procyanidin B1
Catalog No.:BCN6314
CAS No.:20315-25-7
- Procyanidin B2
Catalog No.:BCN6315
CAS No.:29106-49-8
- Procyanidin B4
Catalog No.:BCN0073
CAS No.:29106-51-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23567-23-9 | SDF | Download SDF |
| PubChem ID | 146798 | Appearance | White powder |
| Formula | C30H26O12 | M.Wt | 578.52 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | (+)-Catechin (4α-8)-(+)-catechin; Proanthocyanidin B3 | ||
| Solubility | Soluble in acetone and acetonitrile | ||
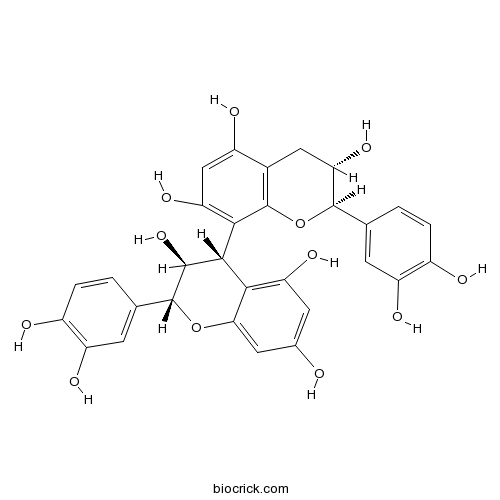
| Chemical Name | (2R,3S)-2-(3,4-dihydroxyphenyl)-8-[(2R,3S,4S)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-4-yl]-3,4-dihydro-2H-chromene-3,5,7-triol | ||
| SMILES | C1C(C(OC2=C1C(=CC(=C2C3C(C(OC4=CC(=CC(=C34)O)O)C5=CC(=C(C=C5)O)O)O)O)O)C6=CC(=C(C=C6)O)O)O | ||
| Standard InChIKey | XFZJEEAOWLFHDH-AVFWISQGSA-N | ||
| Standard InChI | InChI=1S/C30H26O12/c31-13-7-20(37)24-23(8-13)41-29(12-2-4-16(33)19(36)6-12)27(40)26(24)25-21(38)10-17(34)14-9-22(39)28(42-30(14)25)11-1-3-15(32)18(35)5-11/h1-8,10,22,26-29,31-40H,9H2/t22-,26-,27-,28+,29+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Procyanidin B3(B3) is a procyanidin dimer that is widely studied due to its high abundance in the human diet and antioxidant activity. Procyanidin B3 is an inhibitor of histone acetyltransferase, B3 enhances the action of antagonist for prostate cancer cells via inhibition of p300-dependent acetylation of androgen receptor. Procyanidin B3 prevents osteoarthritis (OA) progression and heterotopic cartilage formation, at least in a part through the suppression of iNOS, these results support the potential therapeutic benefits of B3 for treatment of human OA and heterotopic ossification. |
| Targets | NOS | IL Receptor | Androgen Receptor |
| In vitro | Procyanidin B3 prevents articular cartilage degeneration and heterotopic cartilage formation in a mouse surgical osteoarthritis model.[Pubmed: 22629448]PLoS One. 2012;7(5):e37728.Procyanidin B3 (B3) is a procyanidin dimer that is widely studied due to its high abundance in the human diet and antioxidant activity. Here, we evaluated the role of Procyanidin B3 isolated from grape seeds in the maintenance of chondrocytes in vitro and in vivo. |
| In vivo | Osteoarthritis: Procyanidin B3 prevents OA in vivo.[Pubmed: 22744145]Nat Rev Rheumatol. 2012 Jun 29;8(7):369.Procyanidin B3 prevents articular cartilage degeneration and heterotopic cartilage formation in a mouse surgical osteoarthritis model. |
| Kinase Assay | Procyanidin B3, an inhibitor of histone acetyltransferase, enhances the action of antagonist for prostate cancer cells via inhibition of p300-dependent acetylation of androgen receptor.[Pubmed: 20955177]Influence of carbohydrates on the interaction of procyanidin B3 with trypsin.[Pubmed: 21950419]J Agric Food Chem. 2011 Nov 9;59(21):11794-802.The biological properties of procyanidins, in particular their inhibition of digestive enzymes, have received much attention in the past few years. Dietary carbohydrates are an environmental factor that is known to affect the interaction of procyanidins with proteins. This work aimed at understanding the effect of ionic food carbohydrates (polygalacturonic acid, arabic gum, pectin, and xanthan gum) on the interaction between procyanidins and trypsin. Biochem J. 2011 Jan 1;433(1):235-44.Increasing evidence suggests that AR (androgen receptor) acetylation is critical for prostate cancer cell growth. |

Procyanidin B3 Dilution Calculator

Procyanidin B3 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7285 mL | 8.6427 mL | 17.2855 mL | 34.571 mL | 43.2137 mL |
| 5 mM | 0.3457 mL | 1.7285 mL | 3.4571 mL | 6.9142 mL | 8.6427 mL |
| 10 mM | 0.1729 mL | 0.8643 mL | 1.7285 mL | 3.4571 mL | 4.3214 mL |
| 50 mM | 0.0346 mL | 0.1729 mL | 0.3457 mL | 0.6914 mL | 0.8643 mL |
| 100 mM | 0.0173 mL | 0.0864 mL | 0.1729 mL | 0.3457 mL | 0.4321 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hoechst 34580
Catalog No.:BCC1632
CAS No.:23555-00-2
- HOE 32020
Catalog No.:BCC1620
CAS No.:23554-99-6
- Hoechst 33258 analog 3
Catalog No.:BCC1626
CAS No.:23554-98-5
- Daunorubicin HCl
Catalog No.:BCC5083
CAS No.:23541-50-6
- 8-Debenzoylpaeoniflorin
Catalog No.:BCC8787
CAS No.:23532-11-8
- 5-Aza-2'-deoxycytidine
Catalog No.:BCN2169
CAS No.:2353-33-5
- Vomifoliol
Catalog No.:BCN5088
CAS No.:23526-45-6
- (-)-licarin A
Catalog No.:BCN5087
CAS No.:23518-30-1
- 10-Gingerol
Catalog No.:BCN5922
CAS No.:23513-15-7
- 6-Gingerol
Catalog No.:BCN1030
CAS No.:23513-14-6
- 8-Gingerol
Catalog No.:BCN5921
CAS No.:23513-08-8
- Humulon
Catalog No.:BCC8186
CAS No.:23510-81-8
- Clotrimazole
Catalog No.:BCC3755
CAS No.:23593-75-1
- Norisoboldine
Catalog No.:BCN6285
CAS No.:23599-69-1
- H-Phe-pNA
Catalog No.:BCC3010
CAS No.:2360-97-6
- [Arg14,Lys15]Nociceptin
Catalog No.:BCC5781
CAS No.:236098-40-1
- HOE 32021
Catalog No.:BCC1621
CAS No.:23623-06-5
- HOE 33187
Catalog No.:BCC1622
CAS No.:23623-08-7
- Putraflavone
Catalog No.:BCN5089
CAS No.:23624-21-7
- Kaempferol-3-O-galactoside
Catalog No.:BCN3061
CAS No.:23627-87-4
- Boldenone acetate
Catalog No.:BCC8893
CAS No.:2363-59-9
- Z-Asp(OtBu)-OH.DCHA
Catalog No.:BCC2788
CAS No.:23632-70-4
- Triapine
Catalog No.:BCC5112
CAS No.:236392-56-6
- Clerosterol
Catalog No.:BCN2905
CAS No.:2364-23-0
Influence of carbohydrates on the interaction of procyanidin B3 with trypsin.[Pubmed:21950419]
J Agric Food Chem. 2011 Nov 9;59(21):11794-802.
The biological properties of procyanidins, in particular their inhibition of digestive enzymes, have received much attention in the past few years. Dietary carbohydrates are an environmental factor that is known to affect the interaction of procyanidins with proteins. This work aimed at understanding the effect of ionic food carbohydrates (polygalacturonic acid, arabic gum, pectin, and xanthan gum) on the interaction between procyanidins and trypsin. Physical-chemical techniques such as saturation transfer difference-NMR (STD-NMR) spectroscopy, fluorescence quenching, and nephelometry were used to evaluate the interaction process. Using STD-NMR, it was possible to identify the binding of Procyanidin B3 to trypsin. The tested carbohydrates prevented the association of Procyanidin B3 and trypsin by a competition mechanism in which the ionic character of carbohydrates and their ability to encapsulate procyanidins seem crucial leading to a reduction in STD signal and light scattering and to a recovery of the proteins intrinsic fluorescence. On the basis of these results, it was possible to grade the carbohydrates in their aggregation inhibition ability: XG > PA > AG >> PC. These effects may be relevant since the coingestion of procyanidins and ionic carbohydrates are frequent and furthermore since these might negatively affect the antinutritional properties ascribed to procyanidins in the past.
Procyanidin B3, an inhibitor of histone acetyltransferase, enhances the action of antagonist for prostate cancer cells via inhibition of p300-dependent acetylation of androgen receptor.[Pubmed:20955177]
Biochem J. 2011 Jan 1;433(1):235-44.
Increasing evidence suggests that AR (androgen receptor) acetylation is critical for prostate cancer cell growth. In the present study, we identified Pro-B3 (Procyanidin B3) as a specific HAT (histone acetyltransferase) inhibitor. Pro-B3 selectively inhibited the activity of HATs, but not other epigenetic enzymes. Pro-B3 substantially inhibited the p300-mediated AR acetylation, both in vitro and in vivo. Pro-B3 inhibited both p300-dependent and agonist-induced AR transcription. We demonstrate that the p300-mediated AR acetylation is critical for the hormone responsiveness of AR. Interestingly, B3 treatment efficiently enhanced the antagonist activity of flutamide through suppression of p300 HAT activity, demonstrating that relative p300 activity is critical for the antagonist action. Finally, Pro-B3 treatment inhibited acetylation-dependent prostate cell proliferation and expression of cell-cycle control genes, subsequently increasing cell death, indicating the functional importance of AR acetylation for prostate cancer cell growth.
Procyanidin B3 prevents articular cartilage degeneration and heterotopic cartilage formation in a mouse surgical osteoarthritis model.[Pubmed:22629448]
PLoS One. 2012;7(5):e37728.
Osteoarthritis (OA) is a common disease in the elderly due to an imbalance in cartilage degradation and synthesis. Heterotopic ossification (HO) occurs when ectopic masses of endochondral bone form within the soft tissues around the joints and is triggered by inflammation of the soft tissues. Procyanidin B3 (B3) is a procyanidin dimer that is widely studied due to its high abundance in the human diet and antioxidant activity. Here, we evaluated the role of B3 isolated from grape seeds in the maintenance of chondrocytes in vitro and in vivo. We observed that B3 inhibited H(2)O(2)-induced apoptosis in primary chondrocytes, suppressed H(2)O(2)- or IL-1ss-induced nitric oxide synthase (iNOS) production, and prevented IL-1ss-induced suppression of chondrocyte differentiation marker gene expression in primary chondrocytes. Moreover, B3 treatment enhanced the early differentiation of ATDC5 cells. To examine whether B3 prevents cartilage destruction in vivo, OA was surgically induced in C57BL/6J mice followed by oral administration of B3 or vehicle control. Daily oral B3 administration protected articular cartilage from OA and prevented chondrocyte apoptosis in surgically-induced OA joints. Furthermore, B3 administration prevented heterotopic cartilage formation near the surgical region. iNOS protein expression was enhanced in the synovial tissues and the pseudocapsule around the surgical region in OA mice fed a control diet, but was reduced in mice that received B3. Together, these data indicated that in the OA model, B3 prevented OA progression and heterotopic cartilage formation, at least in a part through the suppression of iNOS. These results support the potential therapeutic benefits of B3 for treatment of human OA and heterotopic ossification.


