Procyanidin B1CAS# 20315-25-7 |
- Procyanidin B3
Catalog No.:BCN6316
CAS No.:23567-23-9
- Procyanidin B2
Catalog No.:BCN6315
CAS No.:29106-49-8
- Procyanidin B4
Catalog No.:BCN0073
CAS No.:29106-51-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20315-25-7 | SDF | Download SDF |
| PubChem ID | 130556 | Appearance | Beige powder |
| Formula | C30H26O12 | M.Wt | 578.52 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | (-)-Epicatechin (4β-8)-(+)-catechin; Proanthocyanidin B1 | ||
| Solubility | DMSO : 25 mg/mL (43.21 mM; Need ultrasonic) | ||
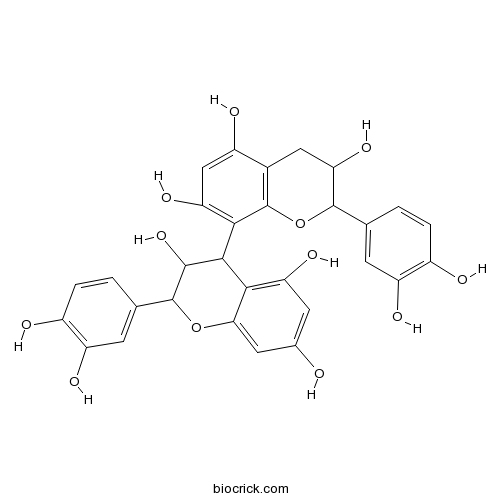
| Chemical Name | 2-(3,4-dihydroxyphenyl)-8-[2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-4-yl]-3,4-dihydro-2H-chromene-3,5,7-triol | ||
| SMILES | C1C(C(OC2=C1C(=CC(=C2C3C(C(OC4=CC(=CC(=C34)O)O)C5=CC(=C(C=C5)O)O)O)O)O)C6=CC(=C(C=C6)O)O)O | ||
| Standard InChIKey | XFZJEEAOWLFHDH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C30H26O12/c31-13-7-20(37)24-23(8-13)41-29(12-2-4-16(33)19(36)6-12)27(40)26(24)25-21(38)10-17(34)14-9-22(39)28(42-30(14)25)11-1-3-15(32)18(35)5-11/h1-8,10,22,26-29,31-40H,9H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Procyanidin B1, a HCV RNA polymerase inhibitor, inhibits infection by vesicular stomatitis virus and HCV pseudotype virus in Huh-7 cells, with IC50 of 29μM and 15μM, respectively. Procyanidin B1 has neuroprotective effects, may attenuate the activation of caspase-3 by inhibiting that of caspase-8 and -9. It has anti-inflammatory effect on LPS-treated THP1 cells via interaction with the TLR4–MD-2 heterodimer and p38 MAPK and NF-κB signaling. |
| Targets | Beta Amyloid | Caspase | HCV | TLR | TNF-α | NF-kB | p38MAPK |
| In vitro | Protective effects of glycycoumarin and procyanidin B1, active components of traditional Japanese medicine yokukansan, on amyloid β oligomer-induced neuronal death.[Pubmed: 25446602]J Ethnopharmacol. 2015 Jan 15;159:122-8.Yokukansan, a traditional Japanese (Kampo) medicine, is composed of seven medicinal herbs, and has been traditionally used to treat neurosis, insomnia, and night crying and irritability in children. Yokukansan and its constituent herbs, Glycyrrhiza and Uncaria Hook, have recently been shown to have protective effects against amyloid β (Aβ) oligomer-induced apoptosis by suppressing the activation of caspase-3 in primary cultured neurons. The aim of the present study was to identify the effective components of Glycyrrhiza and Uncaria Hook against Aβ oligomer-induced neurotoxicity. We also attempted to clarify the mechanisms by which yokukansan and these herbs, as well as their components, suppressed the activation of caspase-3 in Aβ oligomer-treated neurons.
Procyanidin B1 purified from Cinnamomi cortex suppresses hepatitis C virus replication.[Pubmed: 20710064 ]Antivir Chem Chemother. 2010 Aug 11;20(6):239-48.A combination of pegylated interferon and ribavirin is the current standard therapy for hepatitis C virus (HCV) infection, but this combination provides relatively low efficacy, especially in some patients with HCV genotype 1 infection; therefore, the development of novel therapeutic agents is required for further improvement in the treatment of chronic HCV infection.
|
| In vivo | Flavangenol (pine bark extract) and its major component procyanidin B1 enhance fatty acid oxidation in fat-loaded models.[Pubmed: 22227333]Eur J Pharmacol. 2012 Feb 29;677(1-3):147-53.Flavangenol, one of several pine bark extract products, is expected to prevent metabolic diseases with its potent antioxidant effect, its anti-obesity effect and its improvement of insulin sensitivity.
|
| Structure Identification | Molecules. 2014 Feb 4;19(2):1775-85.Inhibitory activity of synthesized acetylated Procyanidin B1 analogs against HeLa S3 cells proliferation.[Pubmed: 24500007]Proanthocyanidins, also known as condensed tannins and/or oligomeric flavonoids, occur in many edible plants and have various interesting biological activities. Previously, we reported a synthetic method for the preparation of various procyanidins in pure form and described their biological activities.
Mol Cell Biochem. 2015 Sep;407(1-2):89-95.Anti-inflammatory effect of procyanidin B1 on LPS-treated THP1 cells via interaction with the TLR4-MD-2 heterodimer and p38 MAPK and NF-κB signaling.[Pubmed: 26037075 ]Anti-inflammatory effects of Procyanidin B1 have been documented; however, the molecular mechanisms that are involved have not been fully elucidated.
|

Procyanidin B1 Dilution Calculator

Procyanidin B1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7285 mL | 8.6427 mL | 17.2855 mL | 34.571 mL | 43.2137 mL |
| 5 mM | 0.3457 mL | 1.7285 mL | 3.4571 mL | 6.9142 mL | 8.6427 mL |
| 10 mM | 0.1729 mL | 0.8643 mL | 1.7285 mL | 3.4571 mL | 4.3214 mL |
| 50 mM | 0.0346 mL | 0.1729 mL | 0.3457 mL | 0.6914 mL | 0.8643 mL |
| 100 mM | 0.0173 mL | 0.0864 mL | 0.1729 mL | 0.3457 mL | 0.4321 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Procyanidin B1 , inhibits infection by vesicular stomatitis virus and HCV pseudotype virus in Huh-7 cells, with IC50 of 29μM and 15μM, respectively. IC50:29μM and 15μM(VSV/HCV infection)[3] In vitro: Procyanidin B1 can bind to the TLR4–MD-2 heterodimer through a competitive interaction with LPS, which suppresses the activation of NF-κB and p38 MAPK pathways. This provide a new insight into the role of procyanidin B1 as an antagonist of the immune response involved in the development and progression of many inflammatory diseases.[1] Procyanidin B1 (PB1), a dimer of (?)-epicatechin and (+)-catechin,suppresses HCV RNA synthesis, possibly as a HCV RNA polymerase inhibitor.No inhibitory effects were observed in each component of PB1. We found that PB1 does not interfere with viral entry or receptor expression, but inhibits HCV RNA synthesis in a dose-dependent manner.[3]
References:
[1]. Xing, J. et al.Anti-inflammatory effect of procyanidin B1 on LPS-treated THP1 cells via interaction with the TLR4-MD-2 heterodimer and p38 MAPK and NF-kappaB signaling. Molecular and cellular biochemistry 407, 89-95, doi:10.1007/s11010-015-2457-4 (2015)
[2]. Terra, X. et al.Procyanidin dimer B1 and trimer C1 impair inflammatory response signalling in human monocytes. Free radical research 45, 611-619, doi:10.3109/10715762.2011.564165 (2011).
[3]. Li, S. et al.Procyanidin B1 purified from Cinnamomi cortex suppresses hepatitis C virus replication. Antiviral chemistry & chemotherapy 20, 239-248, doi:10.3851/IMP1597 (2010).
- Solamargine
Catalog No.:BCN2305
CAS No.:20311-51-7
- Saponarin
Catalog No.:BCN2280
CAS No.:20310-89-8
- Clofazimine
Catalog No.:BCC4651
CAS No.:2030-63-9
- Aporheine
Catalog No.:BCN4802
CAS No.:2030-53-7
- NF 279
Catalog No.:BCC6964
CAS No.:202983-32-2
- NF 340
Catalog No.:BCC7785
CAS No.:202982-98-7
- Conantokin-R
Catalog No.:BCC5980
CAS No.:202925-60-8
- glucagon receptor antagonists 2
Catalog No.:BCC1594
CAS No.:202917-18-8
- glucagon receptor antagonists 3
Catalog No.:BCC1595
CAS No.:202917-17-7
- 7-Benzyloxyindole
Catalog No.:BCC8778
CAS No.:20289-27-4
- 4-Benzyloxyindole
Catalog No.:BCC8700
CAS No.:20289-26-3
- 8-Hydroxy-3,5,7,3',4',5'-hexamethoxyflavone
Catalog No.:BCN1506
CAS No.:202846-95-5
- Tiliroside
Catalog No.:BCN4889
CAS No.:20316-62-5
- Solamarine
Catalog No.:BCN3806
CAS No.:20318-30-3
- 3,5-Diacetamido-4-methylbenzoic acid
Catalog No.:BCN1505
CAS No.:6633-37-0
- 3,4,5-Trimethoxy-trans-cinnamic acid
Catalog No.:BCN3423
CAS No.:20329-98-0
- 3,4-Dimethoxyphenol
Catalog No.:BCN4890
CAS No.:2033-89-8
- H-D-Arg-NH2.2HCl
Catalog No.:BCC2870
CAS No.:203308-91-2
- Daphnoretin
Catalog No.:BCN2473
CAS No.:2034-69-7
- 7-Oxo-beta-sitosterol
Catalog No.:BCN4891
CAS No.:2034-74-4
- Luteollin 5-glucoside
Catalog No.:BCN5391
CAS No.:20344-46-1
- 18-Norabieta-8,11,13-triene-4,15-diol
Catalog No.:BCN1504
CAS No.:203455-81-6
- SNX 482
Catalog No.:BCC5952
CAS No.:203460-30-4
- (+)-Bornyl acetate
Catalog No.:BCN8317
CAS No.:20347-65-3
Flavangenol (pine bark extract) and its major component procyanidin B1 enhance fatty acid oxidation in fat-loaded models.[Pubmed:22227333]
Eur J Pharmacol. 2012 Feb 29;677(1-3):147-53.
Flavangenol, one of several pine bark extract products, is expected to prevent metabolic diseases with its potent antioxidant effect, its anti-obesity effect and its improvement of insulin sensitivity. In this study, targeting the liver as one of the organs that plays an important role in energy metabolism, Flavangenol was investigated for its effect on non-alcoholic fatty liver disease (NAFLD), its action mechanism and its active ingredients, using in vivo and in vitro experiment systems. Flavangenol suppressed intrahepatic fat accumulation in Western diet-loaded Tsumura Suzuki Obese Diabetes (TSOD) mice, which develop various metabolic diseases. In addition, Flavangenol significantly increased the mRNA expression levels of fatty acid oxidative enzymes (peroxisomal proliferator-activated receptor alpha, acyl-CoA oxidase, carnitine palmitoyltransferase). In order to investigate the direct effect of Flavangenol on the liver, an in vitro fatty liver model prepared by adding a free fatty acid to human liver cancer cells (HepG2 cells) was used. In this model, Flavangenol significantly suppressed intracellular fat accumulation. Procyanidin B1, one of the major components of Flavangenol, also suppressed fat accumulation and induced mRNA expression of the fatty acid oxidative enzymes. As mentioned above, Flavangenol showed a significant suppressive effect in the NAFLD model, and it was suggested that the molecular mechanism is induction of fatty acid oxidation, with the effect mainly attributed to Procyanidin B1.
Protective effects of glycycoumarin and procyanidin B1, active components of traditional Japanese medicine yokukansan, on amyloid beta oligomer-induced neuronal death.[Pubmed:25446602]
J Ethnopharmacol. 2015 Jan 15;159:122-8.
ETHNOPHARMACOLOGICAL RELEVANCE: Yokukansan, a traditional Japanese (Kampo) medicine, is composed of seven medicinal herbs, and has been traditionally used to treat neurosis, insomnia, and night crying and irritability in children. Yokukansan and its constituent herbs, Glycyrrhiza and Uncaria Hook, have recently been shown to have protective effects against amyloid beta (Abeta) oligomer-induced apoptosis by suppressing the activation of caspase-3 in primary cultured neurons. The aim of the present study was to identify the effective components of Glycyrrhiza and Uncaria Hook against Abeta oligomer-induced neurotoxicity. We also attempted to clarify the mechanisms by which yokukansan and these herbs, as well as their components, suppressed the activation of caspase-3 in Abeta oligomer-treated neurons. MATERIALS AND METHODS: Rat primary cultured cortical neurons were treated with Abeta oligomer (3 muM). The protective effects of 16 components derived from Glycyrrhiza or Uncaria Hook against Abeta oligomer-induced neurotoxicity were determined using the MTT reduction assay 48 h after the treatment. The suppressive effects of the test substances, i.e., yokukansan, Glycyrrhiza, Uncaria Hook, and screened components, on the Abeta oligomer-induced activation of caspase-3(/7) were evaluated using the caspase-Glo assay 48 h after the Abeta oligomer treatment. The suppressive effects of the test substances on the activation of caspase-8 and -9, both of which are located upstream of caspase-3, were also examined 24h after the Abeta oligomer treatment. RESULTS: Two of the 16 components tested, glycycoumarin derived from Glycyrrhiza and Procyanidin B1 derived from Uncaria Hook, significantly inhibited Abeta oligomer-induced neuronal death in a dose-dependent manner. Glycyrrhiza, Uncaria Hook, and yokukansan significantly suppressed the Abeta oligomer-induced activation of caspase-3 as well as caspase-8 and -9. Glycycoumarin also suppressed the activation of caspase-3, but not caspase-8 and -9. Procyanidin B1 suppressed the activation of caspase-3, -8, and -9. CONCLUSIONS: Our results demonstrated that glycycoumarin and Procyanidin B1 had ameliorative effects on Abeta oligomer-induced neurotoxicity. The neuroprotective effects of glycycoumarin are thought to be due to the attenuated activation of caspase-3, but not caspase-8 or -9. Procyanidin B1, as well as yokukansan, Glycyrrhiza, and Uncaria Hook, may attenuate the activation of caspase-3 by inhibiting that of caspase-8 and -9.
Procyanidin B1 purified from Cinnamomi cortex suppresses hepatitis C virus replication.[Pubmed:20710064]
Antivir Chem Chemother. 2010 Aug 11;20(6):239-48.
BACKGROUND: A combination of pegylated interferon and ribavirin is the current standard therapy for hepatitis C virus (HCV) infection, but this combination provides relatively low efficacy, especially in some patients with HCV genotype 1 infection; therefore, the development of novel therapeutic agents is required for further improvement in the treatment of chronic HCV infection. METHODS: HCV pseudotype and subgenomic replicon assays were used in this study. The interaction of compounds with HCV receptors was examined using flow cytometry. Intracellular RNA levels were determined by semi-quantitative reverse transcriptase PCR. RESULTS: Procyanidin B1 (PB1), a dimer of (-)-epicatechin and (+)-catechin, purified from Cinnamomi cortex, inhibits infection by vesicular stomatitis virus and HCV pseudotype virus in Huh-7 cells, with 50% effective concentrations of 29 and 15 microM, respectively. No inhibitory effects were observed in each component of PB1. We found that PB1 does not interfere with viral entry or receptor expression, but inhibits HCV RNA synthesis in a dose-dependent manner. CONCLUSIONS: These results indicate that PB1 suppresses HCV RNA synthesis, possibly as a HCV RNA polymerase inhibitor. Our results might contribute towards the development of more effective inhibitors for HCV infection from natural plants.
Anti-inflammatory effect of procyanidin B1 on LPS-treated THP1 cells via interaction with the TLR4-MD-2 heterodimer and p38 MAPK and NF-kappaB signaling.[Pubmed:26037075]
Mol Cell Biochem. 2015 Sep;407(1-2):89-95.
Anti-inflammatory effects of Procyanidin B1 have been documented; however, the molecular mechanisms that are involved have not been fully elucidated. Molecular docking models were applied to evaluate the binding capacity of lipopolysaccharide (LPS) and Procyanidin B1 with the toll-like receptor (TLR)4/myeloid differentiation factor (MD)-2 complex. LPS-induced production of the proinflammatory cytokine tumor necrosis factor (TNF)-alpha in a human monocyte cell line (THP1) was measured by ELISA. mRNA expression of MD-2, TLR4, TNF receptor-associated factor (TRAF)-6, and nuclear factor (NF)-kappaB was measured by real-time PCR with or without an 18-h co-treatment with Procyanidin B1. In addition, protein expression of phosphorylated p38 mitogen-activated protein kinase (MAPK) and NF-kappaB was determined by Western blotting. Structural modeling studies identified Tyr296 in TLR4 and Ser120 in MD-2 as critical sites for hydrogen bonding with Procyanidin B1, similar to the sites occupied by LPS. The production of TNF-alpha was significantly decreased by Procyanidin B1 in LPS-treated THP1 cells (p < 0.05). Procyanidin B1 also significantly suppressed levels of phosphorylated p38 MAPK and NF-kappaB protein, as well as mRNA levels of MD-2, TRAF-6, and NF-kappaB (all p < 0.05). Procyanidin B1 can compete with LPS for binding to the TLR4-MD-2 heterodimer and suppress downstream activation of p38 MAPK and NF-kappaB signaling pathways.
Inhibitory activity of synthesized acetylated Procyanidin B1 analogs against HeLa S3 cells proliferation.[Pubmed:24500007]
Molecules. 2014 Feb 4;19(2):1775-85.
Proanthocyanidins, also known as condensed tannins and/or oligomeric flavonoids, occur in many edible plants and have various interesting biological activities. Previously, we reported a synthetic method for the preparation of various procyanidins in pure form and described their biological activities. Here, we describe the synthesis of Procyanidin B1 acetylated analogs and discuss their inhibition activities against HeLa S3 cell proliferation. Surprisingly, the lower-unit acetylated Procyanidin B1 strongly inhibited the proliferation of HeLa S3 cells. This molecule showed much stronger inhibitory activity than did epigallocatechin-3-O-gallate (EGCG), green tea polyphenol, and dimeric compounds that included EGCG as a unit. This result suggests that the phenolic hydroxyl groups of the upper-units in flavan-3-ols are important for their inhibitory activity against cancer cell proliferation and that a hydrophobic lower unit dimer enhances this activity.


