PD168393EGFR inhibitor CAS# 194423-15-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 194423-15-9 | SDF | Download SDF |
| PubChem ID | 4708 | Appearance | Powder |
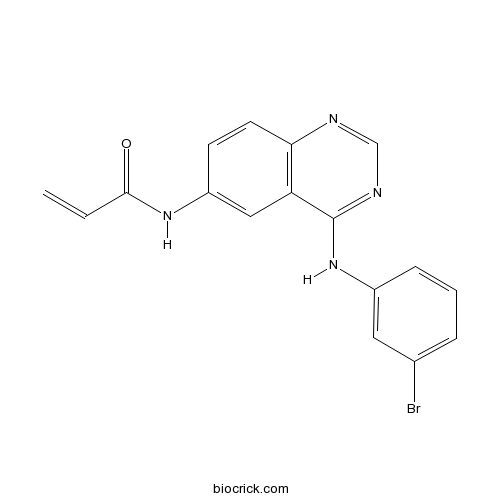
| Formula | C17H13BrN4O | M.Wt | 369.22 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 30 mg/mL (81.25 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N-[4-(3-bromoanilino)quinazolin-6-yl]prop-2-enamide | ||
| SMILES | C=CC(=O)NC1=CC2=C(C=C1)N=CN=C2NC3=CC(=CC=C3)Br | ||
| Standard InChIKey | HTUBKQUPEREOGA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | PD168393 is a potent, cell-permeable, irreversible inhibitor of EGFR with IC50 value of 700 pM. | |||||
| Targets | EGFR | |||||
| IC50 | 700 pM | |||||

PD168393 Dilution Calculator

PD168393 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7084 mL | 13.5421 mL | 27.0841 mL | 54.1682 mL | 67.7103 mL |
| 5 mM | 0.5417 mL | 2.7084 mL | 5.4168 mL | 10.8336 mL | 13.5421 mL |
| 10 mM | 0.2708 mL | 1.3542 mL | 2.7084 mL | 5.4168 mL | 6.771 mL |
| 50 mM | 0.0542 mL | 0.2708 mL | 0.5417 mL | 1.0834 mL | 1.3542 mL |
| 100 mM | 0.0271 mL | 0.1354 mL | 0.2708 mL | 0.5417 mL | 0.6771 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PD168393 is an irreversible kinase inhibitor of epidermal growth factor receptor (EGFR) with IC50 value of 0.70±0.09 nM and continued to have suppressed kinase activity after 8 hr in compound-free medium.[1]
The epidermal growth factor receptor (EGFR; ErbB-1; HER1 in humans) is the cell-surface receptor for members of the epidermal growth factor family (EGF-family) of extracellular protein ligands. Upon activation by its growth factor ligands, EGFR undergoes a transition from an inactive monomeric form to an active homodimer.[2] In addition to forming homodimers after ligand binding, EGFR may pair with another member of the ErbB receptor family to create an activated heterodimer. EGFR dimerization stimulates its intrinsic intracellular protein-tyrosine kinase activity. [3] As a result, autophosphorylation of several tyrosine (Y) residues in the C-terminal domain of EGFR occurs which elicits downstream activation and signaling by several other proteins that associate with the phosphorylated tyrosines through their own phosphotyrosine-binding SH2 domains. These downstream signaling proteins initiate several signal transduction cascades, principally the MAPK, Akt and JNK pathways, leading to DNA synthesis and cell proliferation.[4]
Mutations that lead to EGFR overexpression or overactivity have been associated with a number of cancers, thus many therapeutic approaches are aimed at the EGFR now. PD 168393 can poss a high specificity toward the EGFr with Cys-773 which inhibit the EGFR tyrosine kinase, which is on the cytoplasmic side of the receptor. Without kinase activity, EGFR is unable to activate itself, which is a prerequisite for binding of downstream adaptor proteins. Ostensibly by halting the signaling cascade in cells that rely on this pathway for growth, tumor proliferation and migration is diminished.[5]
PD168393 can enhance paclitaxel-induced DNA fragmentation, sub-G1 fraction accumulation, mitochondrial membrane dysfunction, cytochrome C release, caspase-3 activation and eventually apoptosis in vitro by MTT assay and median-effect analysis. In conclusion, the combination of paclitaxel and PD168393 produced a profound synergistic growth inhibition of AIPC cells,resulting in clinical benefits and warrants further investigation.[6]
References:
1.David W. Fry. et al. Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Pharmacology. Proc Natl Acad Sci U S A. 1998, 95(20): 12022–12027.
2.Yosef Yarden and Joseph Schlessinger. "Epidermal Growth-Factor Induces Rapid, Reversible Aggregation of the Purified Epidermal Growth-Factor Receptor". Biochemistry 1987,26 (5): 1443–1451.
3.Downward J, Parker P, Waterfield MD. "Autophosphorylation sites on the epidermal growth factor receptor". Nature 1984,311 (5985): 483–5.
4.Oda K, Matsuoka Y, Funahashi A, Kitano H. "A comprehensive pathway map of epidermal growth factor receptor signaling". Mol. Syst. Biol,2005,1 (1).
5.Olive DM ."Quantitative methods for the analysis of protein Phosphorylation In drug development". Expert Rev Proteomics2004, 1(3):327–41.
6.Pu YS1, Hsieh MW, et al. Epidermal growth factor receptor inhibitor (PD168393) potentiates cytotoxic effects of paclitaxel against androgen-independent prostate cancer cells. Biochem Pharmacol.
- CL-387785 (EKI-785)
Catalog No.:BCC6436
CAS No.:194423-06-8
- Benzyl Caffeate
Catalog No.:BCC5100
CAS No.:107843-77-6
- Ac-D-Ala-OH
Catalog No.:BCC3196
CAS No.:19436-52-3
- Curcumenol
Catalog No.:BCN3522
CAS No.:19431-84-6
- Selinidin
Catalog No.:BCN2711
CAS No.:19427-82-8
- N-Acetyl-5,6-dehydrololine
Catalog No.:BCN2007
CAS No.:194205-01-1
- Isohyenanchin
Catalog No.:BCN1187
CAS No.:19417-00-6
- Isosaponarin
Catalog No.:BCN2279
CAS No.:19416-87-6
- Rivulobirin B
Catalog No.:BCN1186
CAS No.:194145-29-4
- Dihydrocapsaicin
Catalog No.:BCN1017
CAS No.:19408-84-5
- 3-Amino-4-methylbenzamide
Catalog No.:BCC8614
CAS No.:19406-86-1
- 6,19-Dihydroxyurs-12-en-3-oxo-28-oic acid
Catalog No.:BCN1513
CAS No.:194027-11-7
- Taberpsychine
Catalog No.:BCN4047
CAS No.:19452-84-7
- 24-Norhopa-4(23),22(29)-diene-3β,6β-diol
Catalog No.:BCN1512
CAS No.:194613-74-6
- Rubrosterone
Catalog No.:BCN1511
CAS No.:19466-41-2
- GaTx2
Catalog No.:BCC6326
CAS No.:194665-85-5
- Melittoside
Catalog No.:BCN8413
CAS No.:19467-03-9
- Taxuspine X
Catalog No.:BCN6936
CAS No.:194782-02-0
- Dracoflavan B1
Catalog No.:BCN3589
CAS No.:194794-44-0
- Dracoflavan B2
Catalog No.:BCN3590
CAS No.:194794-47-3
- Dracoflavan C1
Catalog No.:BCN3587
CAS No.:194794-49-5
- Dracoflavan C2
Catalog No.:BCN3586
CAS No.:194794-50-8
- Jujuboside D
Catalog No.:BCN4951
CAS No.:194851-84-8
- Ginsenoside Rs3
Catalog No.:BCN3716
CAS No.:194861-70-6
Epidermal growth factor receptor inhibitor (PD168393) potentiates cytotoxic effects of paclitaxel against androgen-independent prostate cancer cells.[Pubmed:16413505]
Biochem Pharmacol. 2006 Mar 14;71(6):751-60.
Recent data showed that epidermal growth factor receptor (EGFR) inhibitors, such as ZD1839, alone or in combination with chemotherapeutic agents for androgen-independent prostate cancer (AIPC) did not produce promising results in clinical settings. More effective regimens involving novel stronger inhibitor of EGFR and better combinations are needed. The anti-tumor activity of PD168393, an irreversible EGFR inhibitor, with or without chemotherapeutic agents for the treatment of AIPC was investigated in vitro. In results, both the androgen-independent cell lines PC-3 and DU145 expressed higher levels of EGFR than the androgen-dependent MDA PCa 2b and androgen-responsive LNCaP cells by Western blotting. DU145 was much more sensitive to PD168393 and ZD1839 than MDA PCa 2b. PD168393, but not ZD1839, significantly potentiated paclitaxel cytotoxicity against DU145 by MTT assay and median-effect analysis. The combination of PD168393 or ZD1839 with other cytotoxic agents including docetaxel and 5-fluorouracil, however, was either additive or antagonistic. Compared to paclitaxel alone, PD168393 significantly enhanced paclitaxel-induced DNA fragmentation, sub-G1 fraction accumulation, mitochondrial membrane dysfunction, cytochrome C release, caspase-3 activation and eventually apoptosis. These molecular events were accompanied by Bad up-regulation, p53 and p21Waf1/Cip1 induction, ERK1/2 inactivation and inhibition of EGFR phosphorylation in the presence of PD168393. These effects did not involve significant alteration in the Akt1/2 and STAT3 signaling pathway. In conclusion, the combination of paclitaxel and PD168393 produced a profound synergistic growth inhibition of AIPC cells. Combining PD168393 with paclitaxel may have clinical benefits and warrants further investigation.
The pan erbB inhibitor PD168393 enhances lysosomal dysfunction-induced apoptotic death in malignant peripheral nerve sheath tumor cells.[Pubmed:22259051]
Neuro Oncol. 2012 Mar;14(3):266-77.
Malignant peripheral nerve sheath tumors (MPNSTs) are rapidly progressive Schwann cell neoplasms. The erbB family of membrane tyrosine kinases has been implicated in MPNST mitogenesis and invasion and, thus, is a potential therapeutic target. However, tyrosine kinase inhibitors (TKIs) used alone have limited tumoricidal activity. Manipulating the autophagy lysosomal pathway in cells treated with cytostatic agents can promote apoptotic cell death in some cases. The goal of this study was to establish a mechanistic basis for formulating drug combinations to effectively trigger death in MPNST cells. We assessed the effects of the pan erbB inhibitor PD168393 on MPNST cell survival, caspase activation, and autophagy. PD168393 induced a cytostatic but not a cytotoxic response in MPNST cells that was accompanied by suppression of Akt and mTOR activation and increased autophagic activity. The effects of autophagy modulation on MPNST survival were then assessed following the induction of chloroquine (CQ)-induced lysosomal stress. In CQ-treated cells, suppression of autophagy was accompanied by increased caspase activation. In contrast, increased autophagy induction by inhibition of mTOR did not trigger cytotoxicity, possibly because of Akt activation. We thus hypothesized that dual targeting of mTOR and Akt by PD168393 would significantly increase cytotoxicity in cells exposed to lysosomal stress. We found that PD168393 and CQ in combination significantly increased cytotoxicity. We conclude that combinatorial therapies with erbB inhibitors and agents inducing lysosomal dysfunction may be an effective means of treating MPNSTs.
Effects of ROCK inhibitor Y27632 and EGFR inhibitor PD168393 on human neural precursors co-cultured with rat auditory brainstem explant.[Pubmed:25514049]
Neuroscience. 2015 Feb 26;287:43-54.
Hearing function lost by degeneration of inner ear spiral ganglion neurons (SGNs) in the auditory nervous system could potentially be compensated by cellular replacement using suitable donor cells. Donor cell-derived neuronal development with functional synaptic formation with auditory neurons of the cochlear nucleus (CN) in the brainstem is a prerequisite for a successful transplantation. Here a rat auditory brainstem explant culture system was used as a screening platform for donor cells. The explants were co-cultured with human neural precursor cells (HNPCs) to determine HNPCs developmental potential in the presence of environmental cues characteristic for the auditory brainstem region in vitro. We explored effects of pharmacological inhibition of GTPase Rho with its effector Rho-associated kinase (ROCK) and epidermal growth factor receptor (EGFR) signaling on the co-cultures. Pharmacological agents ROCK inhibitor Y27632 and EGFR blocker PD168393 were tested. Effect of the treatment on explant penetration by green fluorescent protein (GFP)-labeled HNPCs was evaluated based on the following criteria: number of GFP-HNPCs located within the explant; distance migrated by the GFP-HNPCs deep into the explant; length of the GFP+/neuronal class III beta-tubulin (TUJ1)+ processes developed and phenotypes displayed. In a short 2-week co-culture both inhibitors had growth-promoting effects on HNPCs, prominent in neurite extension elongation. Significant enhancement of migration and in-growth of HNPCs into the brain slice tissue was only observed in Y27632-treated co-cultures. Difference between Y27632- and PD168393-treated HNPCs acquiring neuronal fate was significant, though not different from the fates acquired in control co-culture. Our data suggest the presence of inhibitory mechanisms in the graft-host environment of the auditory brainstem slice co-culture system with neurite growth arresting properties which can be modulated by administration of signaling pathways antagonists. Therefore the co-culture system can be utilized for screens of donor cells and compounds regulating neuronal fate determination.
A liquid chromatography mass spectrometry assay for determination of PD168393, a specific and irreversible inhibitor of erbB membrane tyrosine kinases, in rat serum.[Pubmed:19010087]
J Chromatogr B Analyt Technol Biomed Life Sci. 2008 Dec 15;876(2):219-24.
For the first time, a rapid, sensitive and simple liquid chromatography/tandem mass spectrometry (LC-MS/MS) method using an atmospheric pressure chemical ionization (APCI) source for the quantification of PD168393 in rat serum was developed and validated. Serum samples were pretreated with methanol for protein precipitation. The chromatographic separation was performed on a Jupiter-C5 column (250 mm x 2.0 mm i.d.) pre-equilibrated with 0.1% formic acid. The tandem mass spectrometer was tuned in the multiple reaction monitoring mode to monitor the m/z transitions 369/313 for PD168393 and m/z 343/308 for the internal standard triazolam, using positive ion mode. The MS/MS response was linear over the concentration range from 2 ng/mL to 5000 ng/mL, with a lower limit of quantification (LLQ) of 2 ng/mL. At the lowest quality control (4 ng/mL), the intra- and inter-day precisions (CV%) for PD168393 were less than 10% and the accuracies were between 92% and 111%. The validated method can be used in most or all stages of the screening and optimizing process for future method validation of pharmacokinetic studies.


