MelittosideCAS# 19467-03-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19467-03-9 | SDF | Download SDF |
| PubChem ID | 11968737 | Appearance | Powder |
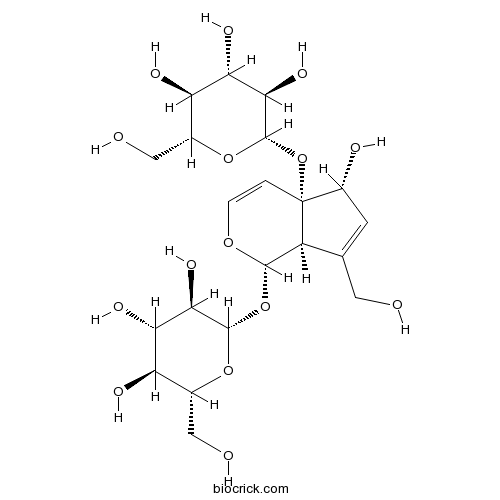
| Formula | C21H32O15 | M.Wt | 524.47 |
| Type of Compound | Iridoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO and methan | ||
| Chemical Name | (2S,3R,4S,5S,6R)-2-[[(1S,4aS,5R,7aR)-5-hydroxy-7-(hydroxymethyl)-4a-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-5,7a-dihydro-1H-cyclopenta[c]pyran-1-yl]oxy]-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | C1=COC(C2C1(C(C=C2CO)O)OC3C(C(C(C(O3)CO)O)O)O)OC4C(C(C(C(O4)CO)O)O)O | ||
| Standard InChIKey | LZKBAGSBRBMVBE-GVKBFFPQSA-N | ||
| Standard InChI | InChI=1S/C21H32O15/c22-4-7-3-10(25)21(36-20-17(31)15(29)13(27)9(6-24)34-20)1-2-32-18(11(7)21)35-19-16(30)14(28)12(26)8(5-23)33-19/h1-3,8-20,22-31H,4-6H2/t8-,9-,10-,11+,12-,13-,14+,15+,16-,17-,18+,19+,20+,21-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Melittoside may have antioxidant activity. |
| Targets | ROS |

Melittoside Dilution Calculator

Melittoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9067 mL | 9.5334 mL | 19.0669 mL | 38.1337 mL | 47.6672 mL |
| 5 mM | 0.3813 mL | 1.9067 mL | 3.8134 mL | 7.6267 mL | 9.5334 mL |
| 10 mM | 0.1907 mL | 0.9533 mL | 1.9067 mL | 3.8134 mL | 4.7667 mL |
| 50 mM | 0.0381 mL | 0.1907 mL | 0.3813 mL | 0.7627 mL | 0.9533 mL |
| 100 mM | 0.0191 mL | 0.0953 mL | 0.1907 mL | 0.3813 mL | 0.4767 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Melittoside is a natural compound.
References:
[1]. Serrilli AM, et al. Monoterpenoids from Stachys glutinosa L. Nat Prod Res. 2006 May 20;20(6):648-52.
[2]. Nan H, et al. A new phenylethanoid glycoside from Clerodendrum inerme. Pharmazie. 2005 Oct;60(10):798-9.
- GaTx2
Catalog No.:BCC6326
CAS No.:194665-85-5
- Rubrosterone
Catalog No.:BCN1511
CAS No.:19466-41-2
- 24-Norhopa-4(23),22(29)-diene-3β,6β-diol
Catalog No.:BCN1512
CAS No.:194613-74-6
- Taberpsychine
Catalog No.:BCN4047
CAS No.:19452-84-7
- PD168393
Catalog No.:BCC1157
CAS No.:194423-15-9
- CL-387785 (EKI-785)
Catalog No.:BCC6436
CAS No.:194423-06-8
- Benzyl Caffeate
Catalog No.:BCC5100
CAS No.:107843-77-6
- Ac-D-Ala-OH
Catalog No.:BCC3196
CAS No.:19436-52-3
- Curcumenol
Catalog No.:BCN3522
CAS No.:19431-84-6
- Selinidin
Catalog No.:BCN2711
CAS No.:19427-82-8
- N-Acetyl-5,6-dehydrololine
Catalog No.:BCN2007
CAS No.:194205-01-1
- Isohyenanchin
Catalog No.:BCN1187
CAS No.:19417-00-6
- Taxuspine X
Catalog No.:BCN6936
CAS No.:194782-02-0
- Dracoflavan B1
Catalog No.:BCN3589
CAS No.:194794-44-0
- Dracoflavan B2
Catalog No.:BCN3590
CAS No.:194794-47-3
- Dracoflavan C1
Catalog No.:BCN3587
CAS No.:194794-49-5
- Dracoflavan C2
Catalog No.:BCN3586
CAS No.:194794-50-8
- Jujuboside D
Catalog No.:BCN4951
CAS No.:194851-84-8
- Ginsenoside Rs3
Catalog No.:BCN3716
CAS No.:194861-70-6
- ACPT-I
Catalog No.:BCC5702
CAS No.:194918-76-8
- Daphnetin 7-methyl ether
Catalog No.:BCN2734
CAS No.:19492-03-6
- 4-Hydroxycinnamamide
Catalog No.:BCN1188
CAS No.:194940-15-3
- Dihydrosenkyunolide C
Catalog No.:BCC8942
CAS No.:195142-72-4
- 4-DAMP
Catalog No.:BCC6661
CAS No.:1952-15-4
Reassessment of Melittis melissophyllum L. subsp. melissophyllum iridoidic fraction.[Pubmed:26131916]
Nat Prod Res. 2016;30(2):218-22.
The analysis of the polar fraction of Melittis melissophyllum L. subsp. melissophyllum led to the identification of several iridoid glycosides: monoMelittoside (1), Melittoside (2), harpagide (3), acetyl-harpagide (4) and ajugoside (5). Compounds 3 and 4 are considered marker compounds for the genus and, as well as compounds 1, 2 and 5, were already evidenced in a previous study on the nominal species. It was noteworthy of the presence of allobetonicoside (6) which was never reported for this genus. The isolation of 6 is very relevant because of its allose residue on the structure. Allose has been often found in the species of the subfamily Lamioideae even if it mostly regarded flavonoids considered of chemotaxonomical relevance for some correlated genera of Lamiaceae. Same as allosyl-glycosidic flavonoids, the presence of allosyl-glycosidic iridoids may also be an additional chemosystematic evidence of botanical relationships among Lamiaceae species and genera.
Polar constituents composition of endemic Sideritis italica (MILL.) GREUTER et BURTER from Central Italy.[Pubmed:23297676]
Nat Prod Res. 2013 Aug;27(15):1408-12.
Endemic Sideritis italica (MILL.) GREUTER et BURTER (Lamiaceae) occurs mainly in Southern Italy and Sicily and has previously only been studied for the essential oil composition. In this paper, we complete the phytochemical study of a sample of S. italica, previously analysed for its volatile constituents, occurring in the Appennino Umbro-Marchigiano (Central Italy), which is the northern border of the areal distribution of the species. The analysis of medium polarity constituents led to the isolation of several glycosides, such as flavonoids, i.e. scutellarein derivatives; phenylethanoids, i.e. verbascoside; and iridoids, i.e. Melittoside and 5-allosyloxy-aucubin, besides the diterpene siderol. The data reported have chemotaxonomic relevance, since they are in contrast with the hypothesis that in Lamiaceae the species producing iridoids do not usually have relevant essential oil production and vice versa.
Acylated flavone glycosides from Veronica.[Pubmed:14599528]
Phytochemistry. 2003 Dec;64(7):1295-301.
A survey of the flavonoid glycosides of selected taxa in the genus Veronica yielded two new acylated 5,6,7,3',4'-pentahydroxyflavone (6-hydroxyluteolin) glycosides and two unusual allose-containing acylated 5,7,8,4'-tetrahydroxyflavone (isoscutellarein) glycosides. The new compounds were isolated from V. liwanensis and V. longifolia and identified using NMR spectroscopy as 6-hydroxyluteolin 4'-methyl ether 7-O-alpha-rhamnopyranosyl(1"'-->2")[6"-O-acetyl-beta-glucopyranoside] and 6-hydroxyluteolin 7-O-(6"-O-(E)-caffeoyl)-beta-glucopyranoside, respectively. Isoscutellarein 7-O-(6"'-O-acetyl)-beta-allopyranosyl(1"'-->2")-beta-glucopyranoside was obtained from both V. intercedens and V. orientalis and its 4'-methyl ether from V. orientalis only. Complete 1H and 13C NMR spectral assignments are presented for both isoscutellarein glycosides. Two iridoid glucosides new to the genus Veronica (Melittoside and globularifolin) were also isolated from V. intercedens.
[Absorption and pharmacokinetics of radix rehmanniae in rats].[Pubmed:24358782]
Yao Xue Xue Bao. 2013 Sep;48(9):1464-70.
In this paper, absorption and pharmacokinetic study of Radix Rehmanniae was studied by liquid chromatography coupled with mass spectrometry method after oral administration to rats. By comparing the chromatograms of ultraviolet, full scan, extracted ion and selective reaction monitoring (SRM) of standard solution, Radix Rehmanniae, blank plasma and rat plasma post drug administration, catalpol and ajugol were found to be the main compounds absorbed from Radix Rehmanniae. Plasma concentrations of aucubin, dihydrocatalpol, rehmannioside A (or rehmannioside B/ Melittoside) and rehmannioside D were very low. Quantitative method for catalpol and aucubin and semi-quantitative method for other compounds in rat plasma were established. The pharmacokinetic study of those absorbed components was conducted after oral administration of 6 g x kg(-1) Radix Rehmanniae water extract to rats. Cmax, t(1/2) and AUC(0-infinity) of catalpol and ajugol were (2349.05 +/- 1438.34) and (104.25 +/- 82.05) ng x mL(-1), (0.86 +/- 0.32) and (0.96 +/- 0.37) h, (4407.58 +/- 2734.89) and (226.66 +/- 188.38) ng x h x mL(-1), respectively. tmax was at 1.00 h for catalpol and ajugol. Both catalpol and ajugol were absorbed and excreted rapidly.
Monoterpenoids from Stachys glutinosa L.[Pubmed:16835100]
Nat Prod Res. 2006 May 20;20(6):648-52.
The iridoidic composition of Stachys glutinosa L. was examined in comparison with the results obtained in the phytochemical studies on S. corsica Pers. The presence of the known harpagide and acetyl-harpagide were showed together with that of a new di-glycosidic iridoid. The structure of this new compound, the 5-allosyloxy-aucubin, was demonstrated by comparison with the spectroscopical data of monoMelittoside and of its 5-O-glucosyl-derivative, the Melittoside. The presence of allose in Stachys genus seems to be a chemotaxonomical character.
Iridoid, phenylethanoid and flavonoid glycosides from Sideritis trojana.[Pubmed:22024633]
Fitoterapia. 2012 Jan;83(1):130-6.
From the MeOH extract of Sideritis trojana, a new iridoid glycoside, 10-O-(E)-feruloylMelittoside (1) was obtained in addition to four known iridoid glycosides [Melittoside (2), 10-O-(E)-p-coumaroylMelittoside (3), stachysosides E (4) and G (5)]. Moreover, five phenylethanoid glycosides [verbascoside (6), isoacteoside (7), lamalboside (8), leonoside A (9), isolavandulifolioside (10), three flavone glycosides (isoscutellarein 7-O-[6'''-O-acetyl-beta-allopyranosyl-(1-->2)]-beta-glucopyranoside (11), 4'-O-methyisoscutellarein 7-O-[6'''-O-acetyl-beta-allopyranosyl-(1-->2)]-beta-glucopyranoside (12), 3'-hydroxy-4'-O-methyisoscutellarein 7-O-[6'''-O-acetyl-beta-allopyranosyl-(1-->2)]-beta-glucopyranoside (13) and a benzylalcohol derivative (di-O-methylcrenatin) were obtained and identified. The structures were elucidated on the basis of NMR and HRMS data. All compounds were tested for their antioxidant activity by in vitro TEAC assay and some of them exhibited moderate activity (0.97-1.44 mM) when compared with the reference compound (quercetin 1.86 mM). Glycosides 6-13, the most active compounds in the TEAC assay, were also tested by flow cytometry to evaluate their ability to affect the levels of reactive oxygen species (ROS) in human prostate cancer cells (PC3).
A new phenylethanoid glycoside from Clerodendrum inerme.[Pubmed:16259134]
Pharmazie. 2005 Oct;60(10):798-9.
A new phenylethanoid glycoside, 2-(3-methoxy-4-hydroxylphenyl) ethyl-O-2",3"-diacetyl-alpha-L-rhamnopyranosyl-(1-->3)-4-O-(E)-feruloyl-beta-D-gl ucopyranoside, was isolated from the aerial parts of Clerodendrum inerme (L.) Gaertn, together with monoMelittoside, Melittoside, inerminoside A1, verbascoside, isoverbascoside, campneoside I. Their structures were determined by spectroscopic methods.


