Taxuspine XCAS# 194782-02-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 194782-02-0 | SDF | Download SDF |
| PubChem ID | 124511126 | Appearance | Powder |
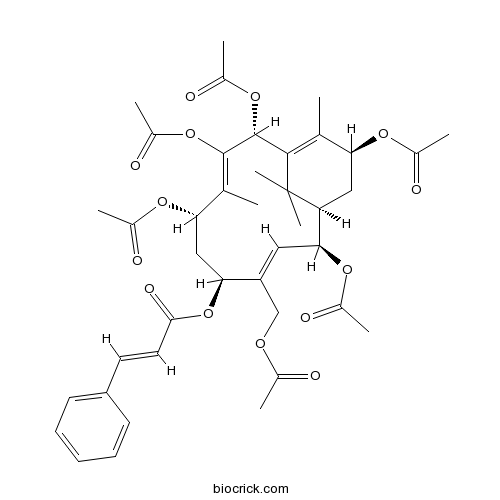
| Formula | C41H50O14 | M.Wt | 766.83 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1R,2S,3E,5S,7S,8Z,10R,13S)-2,7,9,10,13-pentaacetyloxy-4-(acetyloxymethyl)-8,12,15,15-tetramethyl-5-bicyclo[9.3.1]pentadeca-3,8,11-trienyl] (E)-3-phenylprop-2-enoate | ||
| SMILES | CC1=C2C(C(=C(C(CC(C(=CC(C(C2(C)C)CC1OC(=O)C)OC(=O)C)COC(=O)C)OC(=O)C=CC3=CC=CC=C3)OC(=O)C)C)OC(=O)C)OC(=O)C | ||
| Standard InChIKey | FLBNRACKRBUYNP-AHLSXCLRSA-N | ||
| Standard InChI | InChI=1S/C41H50O14/c1-22-33(50-25(4)43)19-32-36(52-27(6)45)18-31(21-49-24(3)42)35(55-37(48)17-16-30-14-12-11-13-15-30)20-34(51-26(5)44)23(2)39(53-28(7)46)40(54-29(8)47)38(22)41(32,9)10/h11-18,32-36,40H,19-21H2,1-10H3/b17-16+,31-18+,39-23-/t32-,33-,34-,35-,36-,40+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Targets | P-gp |

Taxuspine X Dilution Calculator

Taxuspine X Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3041 mL | 6.5204 mL | 13.0407 mL | 26.0814 mL | 32.6018 mL |
| 5 mM | 0.2608 mL | 1.3041 mL | 2.6081 mL | 5.2163 mL | 6.5204 mL |
| 10 mM | 0.1304 mL | 0.652 mL | 1.3041 mL | 2.6081 mL | 3.2602 mL |
| 50 mM | 0.0261 mL | 0.1304 mL | 0.2608 mL | 0.5216 mL | 0.652 mL |
| 100 mM | 0.013 mL | 0.0652 mL | 0.1304 mL | 0.2608 mL | 0.326 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Melittoside
Catalog No.:BCN8413
CAS No.:19467-03-9
- GaTx2
Catalog No.:BCC6326
CAS No.:194665-85-5
- Rubrosterone
Catalog No.:BCN1511
CAS No.:19466-41-2
- 24-Norhopa-4(23),22(29)-diene-3β,6β-diol
Catalog No.:BCN1512
CAS No.:194613-74-6
- Taberpsychine
Catalog No.:BCN4047
CAS No.:19452-84-7
- PD168393
Catalog No.:BCC1157
CAS No.:194423-15-9
- CL-387785 (EKI-785)
Catalog No.:BCC6436
CAS No.:194423-06-8
- Benzyl Caffeate
Catalog No.:BCC5100
CAS No.:107843-77-6
- Ac-D-Ala-OH
Catalog No.:BCC3196
CAS No.:19436-52-3
- Curcumenol
Catalog No.:BCN3522
CAS No.:19431-84-6
- Selinidin
Catalog No.:BCN2711
CAS No.:19427-82-8
- N-Acetyl-5,6-dehydrololine
Catalog No.:BCN2007
CAS No.:194205-01-1
- Dracoflavan B1
Catalog No.:BCN3589
CAS No.:194794-44-0
- Dracoflavan B2
Catalog No.:BCN3590
CAS No.:194794-47-3
- Dracoflavan C1
Catalog No.:BCN3587
CAS No.:194794-49-5
- Dracoflavan C2
Catalog No.:BCN3586
CAS No.:194794-50-8
- Jujuboside D
Catalog No.:BCN4951
CAS No.:194851-84-8
- Ginsenoside Rs3
Catalog No.:BCN3716
CAS No.:194861-70-6
- ACPT-I
Catalog No.:BCC5702
CAS No.:194918-76-8
- Daphnetin 7-methyl ether
Catalog No.:BCN2734
CAS No.:19492-03-6
- 4-Hydroxycinnamamide
Catalog No.:BCN1188
CAS No.:194940-15-3
- Dihydrosenkyunolide C
Catalog No.:BCC8942
CAS No.:195142-72-4
- 4-DAMP
Catalog No.:BCC6661
CAS No.:1952-15-4
- N-(3-Methoxybenzyl)oleamide
Catalog No.:BCC6942
CAS No.:883715-21-7
Taxane diterpenoids from the seeds of Chinese yew Taxus chinensis.[Pubmed:10647221]
Phytochemistry. 1999 Dec;52(8):1565-9.
The taxoid chinentaxunine has been isolated from the seeds of Chinese yew Taxus chinensis, and its structure determined on the basis of spectral and chemical methods. In addition, the known taxol C, paclitaxel, 10-deacetyl taxol A, 10-deacetyl-7-epitaxol, 10-deacetyl-10-oxo-7-epi-taxol, taxinine M, taxchinin A, 10-deacetyl taxinine B and Taxuspine X were also isolated and identified from this source.
From taxuspine x to structurally simplified taxanes with remarkable p-glycoprotein inhibitory activity.[Pubmed:24900226]
ACS Med Chem Lett. 2010 Jul 15;1(8):416-21.
Three simplified "non-natural" natural taxanes, related to Taxuspine X, were synthetized and assayed as P-glycoprotein (P-gp) inhibitors. One of them (6) proved to be a very efficient P-gp inhibitor with an IC50 = 7.2 x 10(-6) M. In addition, to rationalize biological data, a pharmacophoric model was built through a ligand-based approach. This model represents the first example of a pharmacophore, which describes interactions of taxanes with P-gp.
Three new bicyclic taxane diterpenoids from the needles of Japanese yew, Taxus cuspidata Sieb. et Zucc.[Pubmed:11261208]
J Asian Nat Prod Res. 1999;2(1):63-70.
Three new bicyclic taxane diterpenes were isolated from the needles of the Japanese yew, Taxus cuspidata Sieb. et Zucc. Their structures were established to be 7,9,10,13-tetraacetoxy-5-cinnamoyloxy-4-hydroxy-methyl-8,12,15,15- tetramethyl-bicyclo[9.3.1] pentadeca-3,8,11-trien-2-ol (2,20-dideacetyl Taxuspine X), 7,9,10,13,20-pentaacetoxy-5-cinnamoyloxy-8,12,15,15-tetramethyl bicyclo[9.3.1] pentadeca-3,8,11-trien-2-ol (2-deacetyl Taxuspine X), and 9,10,13,20-tetraacetoxy-5-cinnamoyloxy-8,12,15,15-tetramethyl-bicyclo[9.3.1] pentadeca-3,8,11-trien-2,7-diol (2,7-dideacetyl Taxuspine X) with the aid of spectroscopic techniques and by comparing with Taxuspine X.
Taxane diterpenoids from seeds of Taxus mairei.[Pubmed:10993234]
Chem Pharm Bull (Tokyo). 2000 Sep;48(9):1344-6.
A new 2(3-->20) abeotaxane, taxumairone A (1), and a new cis-p-coumaroyl myo-inositol have been isolated from the seeds of Taxus mairei in addition to taxin B (2), taxinine A, Taxuspine X, decinnamoyltaxinine E, 5alpha-cinnamoyloxy-9alpha,10beta,13alpha- triacetoxy-taxa-4(20)11-diene and 5alpha-cinnamoyloxy-2alpha,9alpha,10beta,+ ++13alpha-tetraacetoxy-taxa-4(20)11-diene. The structure of 1 was determined by 2D-NMR spectral analysis and chemical correlation with taxin B (2). Compound 1 exhibited potent cytotoxicity against human colon carcinoma cells with an ED50 of 0.1 microg/ml.
Three novel bicyclic 3,8-secotaxane diterpenoids from the needles of the chinese yew, taxus chinensis var. mairei[Pubmed:9834176]
J Nat Prod. 1998 Nov;61(11):1437-40.
Three novel (1-3) and five known bicyclic 3,8-secotaxane diterpenoids were isolated from the needles of the Chinese yew, Taxus chinensis var. mairei. The structures of the new compounds were established, respectively, as (3E,7E)-2alpha,10beta, 13alpha-triacetoxy-5alpha,20-dihydroxy-3,8-secotaxa-3,7, 11-trien-9-one (1), (3E,7E)-2alpha,10beta-diacetoxy-5alpha,13alpha, 20-trihydroxy-3,8-secotaxa-3,7,11-trien-9-one (2), and (3E,8E)-7beta, 9,10beta,13alpha,20-pentaacetoxy-3,8-secotaxa-3,8,11-triene-2alpha, 5alpha-diol (2-deacetyltaxachitriene A) (3), on the basis of spectral analysis. The other five bicyclic taxane diterpenoids were determined as canadensene, 5-deacetyltaxachitriene B, taxachitriene B, taxuspine U, and Taxuspine X by comparison of their physical properties and spectral data with those previously reported.


