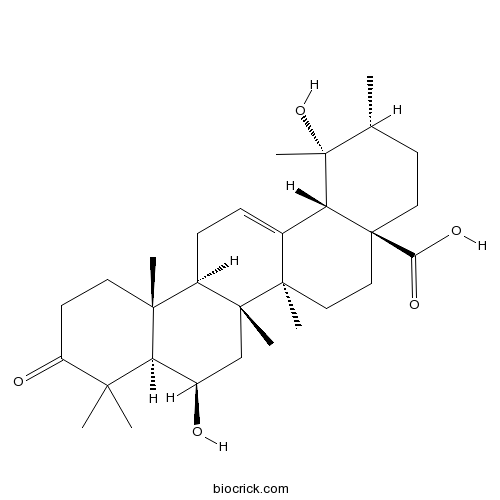
6,19-Dihydroxyurs-12-en-3-oxo-28-oic acidCAS# 194027-11-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 194027-11-7 | SDF | Download SDF |
| PubChem ID | 91895454 | Appearance | Powder |
| Formula | C30H46O5 | M.Wt | 486.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,2R,4aS,6aR,6aS,6bR,8R,8aR,12aR,14bS)-1,8-dihydroxy-1,2,6a,6b,9,9,12a-heptamethyl-10-oxo-3,4,5,6,6a,7,8,8a,11,12,13,14b-dodecahydro-2H-picene-4a-carboxylic acid | ||
| SMILES | CC1CCC2(CCC3(C(=CCC4C3(CC(C5C4(CCC(=O)C5(C)C)C)O)C)C2C1(C)O)C)C(=O)O | ||
| Standard InChIKey | FBFIXJZBTJKFHW-YMRXGCIXSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

6,19-Dihydroxyurs-12-en-3-oxo-28-oic acid Dilution Calculator

6,19-Dihydroxyurs-12-en-3-oxo-28-oic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0547 mL | 10.2733 mL | 20.5465 mL | 41.0931 mL | 51.3663 mL |
| 5 mM | 0.4109 mL | 2.0547 mL | 4.1093 mL | 8.2186 mL | 10.2733 mL |
| 10 mM | 0.2055 mL | 1.0273 mL | 2.0547 mL | 4.1093 mL | 5.1366 mL |
| 50 mM | 0.0411 mL | 0.2055 mL | 0.4109 mL | 0.8219 mL | 1.0273 mL |
| 100 mM | 0.0205 mL | 0.1027 mL | 0.2055 mL | 0.4109 mL | 0.5137 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cedrelopsin
Catalog No.:BCN7687
CAS No.:19397-28-5
- 10-O-Vanilloylaucubin
Catalog No.:BCN1185
CAS No.:193969-08-3
- Fmoc-β-HoPhe-OH
Catalog No.:BCC3241
CAS No.:193954-28-8
- Fmoc- ß-HoIle-OH
Catalog No.:BCC3237
CAS No.:193954-27-7
- Fmoc-ß-HoAla-OH
Catalog No.:BCC3225
CAS No.:193954-26-6
- SC 19220
Catalog No.:BCC6968
CAS No.:19395-87-0
- 3-Phenyl-1,2-dihydroacenaphthylene-1,2-diol
Catalog No.:BCN7178
CAS No.:193892-33-0
- Fmoc-β-Homo-Leu-OH
Catalog No.:BCC2631
CAS No.:193887-44-4
- Tinidazole
Catalog No.:BCC4866
CAS No.:19387-91-8
- Cortistatin 14
Catalog No.:BCC6010
CAS No.:193829-96-8
- SB 242235
Catalog No.:BCC4171
CAS No.:193746-75-7
- Aminoguanidine hydrochloride
Catalog No.:BCC6795
CAS No.:1937-19-5
- 3-Amino-4-methylbenzamide
Catalog No.:BCC8614
CAS No.:19406-86-1
- Dihydrocapsaicin
Catalog No.:BCN1017
CAS No.:19408-84-5
- Rivulobirin B
Catalog No.:BCN1186
CAS No.:194145-29-4
- Isosaponarin
Catalog No.:BCN2279
CAS No.:19416-87-6
- Isohyenanchin
Catalog No.:BCN1187
CAS No.:19417-00-6
- N-Acetyl-5,6-dehydrololine
Catalog No.:BCN2007
CAS No.:194205-01-1
- Selinidin
Catalog No.:BCN2711
CAS No.:19427-82-8
- Curcumenol
Catalog No.:BCN3522
CAS No.:19431-84-6
- Ac-D-Ala-OH
Catalog No.:BCC3196
CAS No.:19436-52-3
- Benzyl Caffeate
Catalog No.:BCC5100
CAS No.:107843-77-6
- CL-387785 (EKI-785)
Catalog No.:BCC6436
CAS No.:194423-06-8
- PD168393
Catalog No.:BCC1157
CAS No.:194423-15-9
Influence of age on intestinal bile acid transport in C57BL/6 mice.[Pubmed:28357119]
Pharmacol Res Perspect. 2017 Feb 3;5(2):e00287.
Intestinal and hepatic bile acid transporters are important for enterohepatic bile acid circulation and pharmacokinetics. Based on previous literature, we hypothesized that the expression of bile acid transporters and intestinal bile acid absorption are lower in older individuals. Here, we measured active taurocholate absorption across the ileum of male C57BL/6 mice in two different age cohorts - young (age range of 89-224 days) and old (age range of 613-953 days). Also examined in these mice were mRNA expression of the major bile acid transporters - Asbt and Ostalpha/beta in the ileum, and Ntcp, Oatp1b2 and Bsep in the liver. Mean intestinal taurocholate absorption was significantly lower (~50%) in mice in the older cohort compared to those in the younger cohort. In the ileum, the expression of Asbt was significantly lower in the older cohort, but expression of Ostalpha/beta was not affected by age. The lower capacity for intestinal bile acid absorption in the older animals is consistent with their lower expression level of Asbt. Of the hepatic bile acid transporters examined, expression of Ntcp and Oatp1b2 were significantly lower in the older mice. This is the first study to directly measure intestinal bile acid absorption as a function of age. The data suggest a lower capacity for intestinal bile acid absorption in older animals. Also, lower expression of Asbt, Ntcp, and Oatp1b2 in older individuals could influence pharmacokinetics of drug substrates.
The N-Acetylmuramic Acid 6-Phosphate Phosphatase MupP Completes the Pseudomonas Peptidoglycan Recycling Pathway Leading to Intrinsic Fosfomycin Resistance.[Pubmed:28351914]
MBio. 2017 Mar 28;8(2). pii: mBio.00092-17.
Bacterial cells are encased in and stabilized by a netlike peptidoglycan (PGN) cell wall that undergoes turnover during bacterial growth. PGN turnover fragments are frequently salvaged by the cells via a pathway referred to as PGN recycling. Two different routes for the recycling of the cell wall sugar N-acetylmuramic acid (MurNAc) have been recognized in bacteria. In Escherichia coli and related enterobacteria, as well as in most Gram-positive bacteria, MurNAc is recovered via a catabolic route requiring a MurNAc 6-phosphate etherase (MurQ in E. coli) enzyme. However, many Gram-negative bacteria, including Pseudomonas species, lack a MurQ ortholog and use an alternative, anabolic recycling route that bypasses the de novo biosynthesis of uridyldiphosphate (UDP)-MurNAc, the first committed precursor of PGN. Bacteria featuring the latter pathway become intrinsically resistant to the antibiotic fosfomycin, which targets the de novo biosynthesis of UDP-MurNAc. We report here the identification and characterization of a phosphatase enzyme, named MupP, that had been predicted to complete the anabolic recycling pathway of Pseudomonas species but has remained unknown so far. It belongs to the large haloacid dehalogenase family of phosphatases and specifically converts MurNAc 6-phosphate to MurNAc. A DeltamupP mutant of Pseudomonas putida was highly susceptible to fosfomycin, accumulated large amounts of MurNAc 6-phosphate, and showed lower levels of UDP-MurNAc than wild-type cells, altogether consistent with a role for MupP in the anabolic PGN recycling route and as a determinant of intrinsic resistance to fosfomycin.IMPORTANCE Many Gram-negative bacteria, but not E. coli, make use of a cell wall salvage pathway that contributes to the pool of UDP-MurNAc, the first committed precursor of cell wall synthesis in bacteria. This salvage pathway is of particular interest because it confers intrinsic resistance to the antibiotic fosfomycin, which blocks de novo UDP-MurNAc biosynthesis. Here we identified and characterized a previously missing enzyme within the salvage pathway, the MurNAc 6-phosphate phosphatase MupP of P. putida MupP, together with the other enzymes of the anabolic recycling pathway, AnmK, AmgK, and MurU, yields UDP-MurNAc, renders bacteria intrinsically resistant to fosfomycin, and thus may serve as a novel drug target for antimicrobial therapy.
Return of the lysergamides. Part III: Analytical characterization of N(6) -ethyl-6-norlysergic acid diethylamide (ETH-LAD) and 1-propionyl ETH-LAD (1P-ETH-LAD).[Pubmed:28342178]
Drug Test Anal. 2017 Oct;9(10):1641-1649.
The psychoactive properties of lysergic acid diethylamide (LSD) have fascinated scientists across disciplines and the exploration of other analogues and derivatives has been motivated by deepening the understanding of ligand-receptor interactions at the molecular level as well as by the search for new therapeutics. Several LSD congeners have appeared on the new psychoactive substances (NPS) market in the form of blotters or powders. Examples include 1-propionyl-LSD (1P-LSD), AL-LAD, and LSZ. The absence of analytical data for novel compounds is a frequent challenge encountered in clinical and toxicological investigations. Two newly emerging lysergamides, namely N(6) -ethyl-6-norlysergic acid diethylamide (ETH-LAD) and 1P-ETH-LAD, were characterized by gas chromatography-mass spectrometry (GC-MS), low and high mass accuracy electrospray MS(/MS), GC solid-state infrared analysis, high performance liquid chromatography diode array detection as well as nuclear magnetic resonance spectroscopy. Limited analytical data for ETH-LAD were previously available, whereas information about 1P-ETH-LAD has not previously been encountered in the scientific literature. This study extends the characterization of lysergamides distributed on the NPS market, which will help to make analytical data available to clinicians, toxicologists, and other stakeholders who are likely to encounter these substances. The analysis of a test incubation of 1P-ETH-LAD with human serum at 37 degrees C by LC single quadrupole MS at various time points (0-6 h, once per hour and one measurement after 24 h) revealed the formation of ETH-LAD, suggesting that 1P-ETH-LAD might serve as a pro-drug. 1P-ETH-LAD was still detectable in serum after 24 h. Copyright (c) 2017 John Wiley & Sons, Ltd.
Effects of humanin on experimental colitis induced by 2,4,6-trinitrobenzene sulphonic acid in rats.[Pubmed:28361841]
Saudi J Gastroenterol. 2017 Mar-Apr;23(2):105-111.
BACKGROUND/AIM: The excessive apoptosis of intestinal epithelial cells (IECs) partly accounts for the development of colonic inflammation and eventually results in ulcerative colitis (UC). Humanin, an endogenous anti-apoptotic peptide, has previously been shown to protect against Alzheimer's disease and a variety of cellular insults. The present study aimed to investigate the effects of glysin variant of humanin (HNG) on 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced colitis in rats. MATERIALS AND METHODS: Rats were divided into four groups as follows: Group 1 (n = 8): control; isotonic saline solution 0.1 ml/rat rectally, Group 2 (n = 8): TNBS colitis; 0.1 ml of a 2.5% (w/v) TNBS solution in 50% ethanol rectally, Group 3 (n = 8): 10 muM HNG, and Group 4 (n = 8): 20 muM HNG intraperitoneal (ip) on day 2 and 6 after rectal TNBS administration. Rats were sacrificed 7 days after the induction of colitis. Blood and tissue samples were harvested for biochemical and histopathological analysis. RESULTS: HNG treatment significantly ameliorated weight loss and macroscopic and microscopic scores. TNBS-induced colitis significantly increased the colonic mRNA expression of tumor necrosis factor-alpha (TNF-alpha), interleukin-1beta (IL-1beta), and caspase-3 activities in group II in comparison to the group I. HNG treatment was associated with an inhibition of mRNA expression of TNF-alpha and IL-1beta, and a decrease in caspase-3 activities in colon tissues in group III and IV when compared to group II. CONCLUSION: The results of this study indicate that HNG treatment may exert beneficial effects in UC by decreasing inflammatory reactions and apoptosis.


