Ouabain OctahydrateCAS# 11018-89-6 |
- RVX-208
Catalog No.:BCC4475
CAS No.:1044870-39-4
- GSK 525768A
Catalog No.:BCC1603
CAS No.:1260530-25-3
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- (-)-JQ1
Catalog No.:BCC3603
CAS No.:1268524-71-5
- MS436
Catalog No.:BCC4037
CAS No.:1395084-25-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

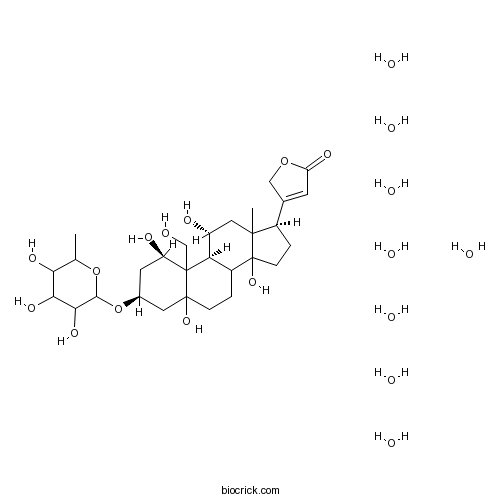
| Cas No. | 11018-89-6 | SDF | Download SDF |
| PubChem ID | 25442 | Appearance | Powder |
| Formula | C29H44O12 | M.Wt | 584.65 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Acocantherine; G-Strophanthin | ||
| Solubility | >19.5mg/ml in H20 | ||
| Chemical Name | 3-[(1R,3S,9S,11R,17R)-1,5,11,14-tetrahydroxy-10-(hydroxymethyl)-13-methyl-3-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy-2,3,4,6,7,8,9,11,12,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl]-2H-furan-5-one;octahydrate | ||
| SMILES | CC1C(C(C(C(O1)OC2CC(C3(C4C(CCC3(C2)O)C5(CCC(C5(CC4O)C)C6=CC(=O)OC6)O)CO)O)O)O)O.O.O.O.O.O.O.O.O | ||
| Standard InChIKey | TYBARJRCFHUHSN-HMNHGHQSSA-N | ||
| Standard InChI | InChI=1S/C29H44O12.8H2O/c1-13-22(34)23(35)24(36)25(40-13)41-15-8-19(32)28(12-30)21-17(3-5-27(28,37)9-15)29(38)6-4-16(14-7-20(33)39-11-14)26(29,2)10-18(21)31;;;;;;;;/h7,13,15-19,21-25,30-32,34-38H,3-6,8-12H2,1-2H3;8*1H2/t13?,15-,16+,17?,18+,19+,21+,22?,23?,24?,25?,26?,27?,28?,29?;;;;;;;;/m0......../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ouabain Octahydrate is an inhibitor of Na+/K+-ATPase, used for the treatment of congestive heart failure.In Vitro:Ouabain (100 μM) induces NLRP3 inflammasome activation and IL-1β release in macrophages. Ouabain-induced NLRP3 inflammasome activation is mediated through K+ efflux[1]. Ouabain (3 nM) alters the expression of EMT markers in NHK and ADPKD cells, and modifies cell-cell adhesion properties in ADPKD. Moreover, ouabain enhances migration of ADPKD cells, selectively modulates tight junctions, and modulates adherens junctions in ADPKD cells in a selective manner. Ouabain also activates TGFβ-Smad3 signaling, alters TER in ADPKD cells[2]. Ouabain (25, 50 or 100 nM) treatment significantly reduces cell proliferation and viability in Raji cells in a dose-dependent manner, with IC50 of 76.48±4.03 nM. Ouabain increases the number of apoptotic cells, induces autophagy, and upregulates Beclin-1 in Raji cells[4].In Vivo:Ouabain (3 mg/kg) significantly decreases cardiac contractile force with an enlarged LVESD when mice are primed with LPS. IL-1β deficiency attenuates ouabain-induced cardiac dysfunction and injury. IL-1β secreted by infiltrated macrophages contributes to ouabain-induced cardiac inflammation. Deficiency of NLRP3 and Casp1 attenuates ouabain-induced cardiac dysfunction and macrophage infiltration[1]. Ouabain (30 µg/kg, i.p.) modulates ABCB1 activity in thymocytes of Wistar rats and it has the same effect on Swiss mice at 300 µg/kg. After 14 days of ouabain treatment, the MAP of rats is significantly elevated[3]. References: | |||||

Ouabain Octahydrate Dilution Calculator

Ouabain Octahydrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7104 mL | 8.5521 mL | 17.1043 mL | 34.2085 mL | 42.7606 mL |
| 5 mM | 0.3421 mL | 1.7104 mL | 3.4209 mL | 6.8417 mL | 8.5521 mL |
| 10 mM | 0.171 mL | 0.8552 mL | 1.7104 mL | 3.4209 mL | 4.2761 mL |
| 50 mM | 0.0342 mL | 0.171 mL | 0.3421 mL | 0.6842 mL | 0.8552 mL |
| 100 mM | 0.0171 mL | 0.0855 mL | 0.171 mL | 0.3421 mL | 0.4276 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ouabain Octahydrate inhibits Na(+)/K(+) ATPase, regulates transcription of MDR and MRP.
- 4-Galloylquinic acid
Catalog No.:BCN3733
CAS No.:110170-37-1
- Methyl hesperidin
Catalog No.:BCN6341
CAS No.:11013-97-1
- Indoximod (NLG-8189)
Catalog No.:BCC5584
CAS No.:110117-83-4
- Ascomycin
Catalog No.:BCN8286
CAS No.:11011-38-4
- des-His1-[Glu9]-Glucagon (1-29) amide
Catalog No.:BCC5885
CAS No.:110084-95-2
- Plerixafor (AMD3100)
Catalog No.:BCC1158
CAS No.:110078-46-1
- 12-Epinapelline
Catalog No.:BCN2800
CAS No.:110064-71-6
- 7-Hydroxy-3-(4-hydroxybenzylidene)chroman-4-one
Catalog No.:BCN1624
CAS No.:110064-50-1
- Strophantin K (mixture)
Catalog No.:BCC8256
CAS No.:11005-63-3
- Calpain Inhibitor I, ALLN
Catalog No.:BCC1233
CAS No.:110044-82-1
- Tussilagonone
Catalog No.:BCC8365
CAS No.:110042-38-1
- Taurohyodeoxycholic Acid Sodium Salt
Catalog No.:BCC8363
CAS No.:110026-03-4
- JZL184
Catalog No.:BCC4790
CAS No.:1101854-58-3
- 1,5,8-Trihydroxy-3-methoxy-2-prenylxanthone
Catalog No.:BCN1623
CAS No.:110187-11-6
- Malonylginsenoside Rb(1)
Catalog No.:BCC9230
CAS No.:88140-34-5
- Cochliophilin A
Catalog No.:BCC8154
CAS No.:110204-45-0
- Ginsenoside Rb2
Catalog No.:BCN1064
CAS No.:11021-13-9
- Ginsenoside Rc
Catalog No.:BCN1072
CAS No.:11021-14-0
- Temocapril HCl
Catalog No.:BCC5016
CAS No.:110221-44-8
- Nothofagin
Catalog No.:BCN3787
CAS No.:11023-94-2
- Digitonin
Catalog No.:BCN3734
CAS No.:11024-24-1
- (-)-beta-Peltatin-5-O-beta-D-glucopyranoside
Catalog No.:BCN3607
CAS No.:11024-59-2
- Ganoderic acid N
Catalog No.:BCN2438
CAS No.:110241-19-5
- Ganoderenic acid E
Catalog No.:BCN8241
CAS No.:110241-23-1
Assessing Teratogenicity from the Clustering of Abnormal Phenotypes in Individual Zebrafish Larvae.[Pubmed:27560445]
Zebrafish. 2016 Dec;13(6):511-522.
In previous publications, we described the population incidence of abnormalities in zebrafish larvae exposed to toxicants. Here, we examine the phenomenon of clustering or co-occurrence of abnormalities in individual larva. Our aim is to see how this clustering can be used to assess the specificity and severity of teratogenic effect. A total of 11,214 surviving larvae, exposed continuously from 1 day postfertilization (dpf) to one of 60 toxicants, were scored at 5 dpf for the presence of eight different abnormal phenotypes. These were as follows: pericardial edema, yolk sac edema, dispersed melanocytes, bent tail, bent trunk, hypoplasia of Meckel's cartilage, hypoplasia of branchial arches, and uninflated swim bladder. For 43/60 compounds tested, there was a concentration-dependent increase in the severity score (number of different abnormalities per larva). Statistical analysis showed that abnormalities tended to cluster (i.e., to occur in the same larva) more often than expected by chance alone. Yolk sac edema and dispersed melanocytes show a relatively strong association with one another and were typically the first abnormalities to appear in single larvae as the concentration of compound was increased. By contrast, hypoplastic branchial arches and hypoplastic Meckel's cartilage were only frequently observed in the most severely affected larvae. We developed a metric of teratogenicity (TC3/8), which represents the concentration of a compound that produces, on average, 3/8 abnormalities per larva. On this basis, the most teratogenic compounds tested here are amitriptyline, chlorpromazine hydrochloride, and sodium dodecyl sulfate; the least teratogenic is ethanol. We find a strong correlation between TC3/8 and LC50 of the 43 compounds that showed teratogenic effects. When we examined the ratio of TC3/8 to LC50, benserazide hydrochloride, copper (II) nitrate trihydrate, and nicotine had the highest specific teratogenicity, while aconitine, hesperidin, and Ouabain Octahydrate had the lowest. We conclude that analyzing the clustering of abnormalities per larva can provide an enriched teratogenic dataset compared with simple measurement of the population frequency of abnormalities.
Anti-HSV activity of digitoxin and its possible mechanisms.[Pubmed:18353452]
Antiviral Res. 2008 Jul;79(1):62-70.
Herpes simplex virus type 1 (HSV-1) can establish latent infection in the nervous system and usually leads to life-threatening diseases in immunocompromised individuals upon reactivation. Treatment with conventional nucleoside analogue such as acyclovir is effective in most cases, but drug-resistance may arise due to prolonged treatment in immunocompromised individuals. In this study, we identified an in-use medication, digitoxin, which actively inhibited HSV-1 replication with a 50% effective concentration (EC(50)) of 0.05 microM. The 50% cytotoxicity concentration (CC(50)) of digitoxin is 10.66 microM and the derived selective index is 213. Several structural analogues of digitoxin such as digoxin, Ouabain Octahydrate and G-strophanthin also showed anti-HSV activity. The inhibitory effects of digitoxin are likely to be introduced at the early stage of HSV-1 replication and the virus release stage. The observation that digitoxin can inhibit acyclovir-resistant viruses further implicates that digitoxin represents a novel drug class with distinct antiviral mechanisms from traditional drugs.
Cocaine suppression of triggered activity. A possible mechanism of antiarrhythmic action.[Pubmed:8120476]
J Electrocardiol. 1994 Jan;27(1):35-9.
The cellular mechanisms of cocaine-induced arrhythmias are not clear. Production of early afterdepolarizations (EADs) in single myocytes have been proposed as a possible mechanism of cocaine arrhythmogenesis. The objective of this investigation was to determine whether cocaine exerts arrhythmogenic or antiarrhythmic actions related to the production or inhibition of afterdepolarizations in multicellular preparations. Rat and guinea pig papillary muscles superfused in vitro with Tyrode's solution at 37 degrees C were stimulated at 1 Hz. Standard intracellular microelectrodes were used to record membrane potentials. Cocaine administered at 2.9-58 microM never induced EADs; also, overdrive in the presence of cocaine never induced delayed afterdepolarizations (DADs). On the other hand, cocaine administered at 58 microM suppressed DADs and triggered activity induced by overdrive in the presence of Ouabain Octahydrate and isoproterenol. Thus, cocaine acting on multicellular ventricular preparations in vitro did not induce EADs or DADs. On the contrary, cocaine inhibited DADs and triggered activity induced by other drugs.
Testing for circadian differences in lethality for intravenous ouabain in male mice.[Pubmed:8258972]
J Ethnopharmacol. 1993 Aug;39(3):161-6.
Using a randomized, balanced design and double-blind methodology, Ouabain Octahydrate was administered intravenously to male mice at six clock times. Eight runs were conducted using six constant dosage levels. All the dose-response curves at the clock times of 02:30, 06:30, 10:30, 14:30, 18:30 and 22:30 were parallel and no significant differences were noted between the respective LD50 determinations using nomograph methods. Independent chi-square analysis of all lethality data indicated no significant variation in response between clock times and between runs but a very highly significant difference between doses. Using regression methods, onset time for death was shown to vary inversely with log-dosage, but those periods of possible increased susceptibility could not be correlated with a shortened time to death. These findings are consistent with a random variation in lethality in regard to clock time rather than a true circadian pattern. The pooled (N = 576) intravenous LD50 for Ouabain Octahydrate was 3.75 mg/kg with 95% confidence limits of 3.60-3.90 mg/kg or, when calculated as anhydrous ouabain, 3.01 (2.89-3.13) mg/kg.


