Indoximod (NLG-8189)Indoleamine 2,3-dioxygenase (IDO) pathway inhibitor Potent and selective HDAC6 inhibitor CAS# 110117-83-4 |
- FK 3311
Catalog No.:BCC1576
CAS No.:116686-15-8
- Iguratimod
Catalog No.:BCC1641
CAS No.:123663-49-0
- Celecoxib
Catalog No.:BCC1099
CAS No.:169590-42-5
- Etoricoxib
Catalog No.:BCC1565
CAS No.:202409-33-4
- Ibuprofen Lysine
Catalog No.:BCC2547
CAS No.:57469-77-9
Quality Control & MSDS
Number of papers citing our products

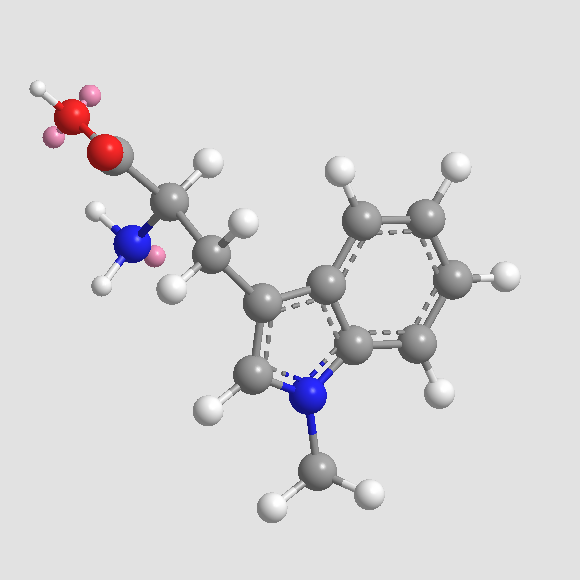
Chemical structure
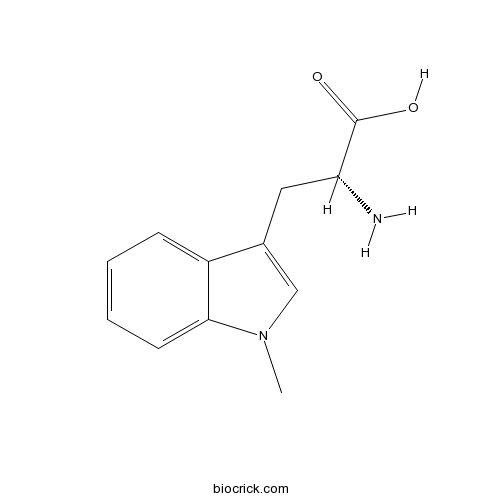
3D structure
| Cas No. | 110117-83-4 | SDF | Download SDF |
| PubChem ID | 405012 | Appearance | Powder |
| Formula | C12H14N2O2 | M.Wt | 218.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 1MT | ||
| Solubility | H2O : 5 mg/mL (22.91 mM; ultrasonic and adjust pH to 2 with HCl) DMSO : 0.55 mg/mL (2.52 mM; Need ultrasonic and warming) | ||
| Chemical Name | (2R)-2-amino-3-(1-methylindol-3-yl)propanoic acid | ||
| SMILES | CN1C=C(C2=CC=CC=C21)CC(C(=O)O)N | ||
| Standard InChIKey | ZADWXFSZEAPBJS-SNVBAGLBSA-N | ||
| Standard InChI | InChI=1S/C12H14N2O2/c1-14-7-8(6-10(13)12(15)16)9-4-2-3-5-11(9)14/h2-5,7,10H,6,13H2,1H3,(H,15,16)/t10-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Indoleamine 2,3-dioxygenase (IDO) inhibitor (IC50 = 7 μM); disrupts tryptophan catabolism. Enhances the antitumor and antiviral immunoresponses of CD8+ T-cells in vitro. Reduces tumor volume in mice with xenografts overexpressing IDO. |

Indoximod (NLG-8189) Dilution Calculator

Indoximod (NLG-8189) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5819 mL | 22.9095 mL | 45.819 mL | 91.638 mL | 114.5475 mL |
| 5 mM | 0.9164 mL | 4.5819 mL | 9.1638 mL | 18.3276 mL | 22.9095 mL |
| 10 mM | 0.4582 mL | 2.291 mL | 4.5819 mL | 9.1638 mL | 11.4548 mL |
| 50 mM | 0.0916 mL | 0.4582 mL | 0.9164 mL | 1.8328 mL | 2.291 mL |
| 100 mM | 0.0458 mL | 0.2291 mL | 0.4582 mL | 0.9164 mL | 1.1455 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Indoximod is an Indoleamine 2,3-dioxygenase (IDO) pathway inhibitor. IDO is a tryptophan-catabolizing enzyme that tumors use to create a state of immunosuppression. [1]
The immunosuppressive activity of IDO leads to an increase in the number of T-regulatory cells, as measured by their Foxp3+/CD4+/CD25+ phenotype. Indoximod has also been shown to reduce the number of T-regulatory cells [2]. In MMTV-Neu mice, researchers looked at the activity of indoximod with and without paclitaxel [3]. The combination of docetaxel and indoximod is more toxic than docetaxel monotherapy. A single 1200 mg dose of indoximod almost totally saturates the gut, and higher doses do not significantly increase peak serum levels. The single-agent phase I trial of indoximod demonstrated very good oral bioavailability and a mild toxicity profile with no significant myelosuppression, and no maximally tolerated dose was identified up to 2000 mg orally twice daily [1].
Blockade of IDO with indoximod enhanced the adoptive immunologic response to antigens and dendritic cell (DC) vaccines in LLC mouse models.
References:
[1]. Soliman HH, Jackson E, Neuger T et al. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget. 2014 Sep 30;5 (18):8136-46.
[2]. Munn DH. Blocking IDO activity to enhance anti-tumor immunity. Front Biosci (Elite Ed). 2012 Jan 1;4:734-45.
[3]. Muller AJ, DuHadaway JB, Donover PS et al.Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005 Mar;11(3):312-9. Epub 2005 Feb 13.
- Ascomycin
Catalog No.:BCN8286
CAS No.:11011-38-4
- des-His1-[Glu9]-Glucagon (1-29) amide
Catalog No.:BCC5885
CAS No.:110084-95-2
- Plerixafor (AMD3100)
Catalog No.:BCC1158
CAS No.:110078-46-1
- 12-Epinapelline
Catalog No.:BCN2800
CAS No.:110064-71-6
- 7-Hydroxy-3-(4-hydroxybenzylidene)chroman-4-one
Catalog No.:BCN1624
CAS No.:110064-50-1
- Strophantin K (mixture)
Catalog No.:BCC8256
CAS No.:11005-63-3
- Calpain Inhibitor I, ALLN
Catalog No.:BCC1233
CAS No.:110044-82-1
- Tussilagonone
Catalog No.:BCC8365
CAS No.:110042-38-1
- Taurohyodeoxycholic Acid Sodium Salt
Catalog No.:BCC8363
CAS No.:110026-03-4
- 8-O-Ethylyunaconitine
Catalog No.:BCN6260
CAS No.:110011-77-3
- Sorbic acid
Catalog No.:BCN2218
CAS No.:110-44-1
- Fumaric acid
Catalog No.:BCN5989
CAS No.:110-17-8
- Methyl hesperidin
Catalog No.:BCN6341
CAS No.:11013-97-1
- 4-Galloylquinic acid
Catalog No.:BCN3733
CAS No.:110170-37-1
- Ouabain Octahydrate
Catalog No.:BCC5211
CAS No.:11018-89-6
- JZL184
Catalog No.:BCC4790
CAS No.:1101854-58-3
- 1,5,8-Trihydroxy-3-methoxy-2-prenylxanthone
Catalog No.:BCN1623
CAS No.:110187-11-6
- Malonylginsenoside Rb(1)
Catalog No.:BCC9230
CAS No.:88140-34-5
- Cochliophilin A
Catalog No.:BCC8154
CAS No.:110204-45-0
- Ginsenoside Rb2
Catalog No.:BCN1064
CAS No.:11021-13-9
- Ginsenoside Rc
Catalog No.:BCN1072
CAS No.:11021-14-0
- Temocapril HCl
Catalog No.:BCC5016
CAS No.:110221-44-8
- Nothofagin
Catalog No.:BCN3787
CAS No.:11023-94-2
- Digitonin
Catalog No.:BCN3734
CAS No.:11024-24-1
Effects of indoleamine 2,3-dioxygenase inhibitor in non-Hodgkin lymphoma model mice.[Pubmed:26243621]
Int J Hematol. 2015 Sep;102(3):327-34.
Indoleamine 2,3-dioxygenase (IDO) catalyzes the rate-limiting step in the metabolism of tryptophan along the kynurenine pathway. In tumors, increased IDO activity inhibits proliferation and induces apoptosis of T cells and natural killer cells. We investigated the therapeutic potential of IDO inhibitor 1-methyl-D-tryptophan (D-1MT) with cyclophosphamide (CY) in a mouse model of lymphoma. To examine the effect of D-1MT, mice were killed on day 28. Serum concentrations of L-kynurenine and L-tryptophan were measured by high-performance liquid chromatography. Regulatory T cells (Tregs) were counted by flow cytometry, and mRNA expressions of IDO1, Foxp3, IFN-gamma, and COX-2 were examined by quantitative real-time reverse transcription-polymerase chain reaction. D-1MT+CY combination treatment significantly inhibited tumor growth as compared to either treatment alone. There were no significant differences in the serum L-kynurenine/L-tryptophan ratio or the IDO1 expression level in the tumors among the treatment groups. The expression levels of IFN-gamma and COX-2 mRNA in tumor-draining lymph nodes (TDLNs) were found to be significantly up-regulated in the CY and D-1MT+CY groups. The number of Tregs in TDLNs in the D-1MT+CY group was significantly lower than that in CY groups on day 17. These results suggest that D-1MT in combination with CY is an effective treatment for lymphoma in a mouse model.
Suppression of immunodominant antitumor and antiviral CD8+ T cell responses by indoleamine 2,3-dioxygenase.[Pubmed:24587363]
PLoS One. 2014 Feb 28;9(2):e90439.
Indoleamine 2,3-dioxygenase (IDO) is a tryptophan-degrading enzyme known to suppress antitumor CD8(+) T cells (TCD8). The role of IDO in regulation of antiviral TCD8 responses is far less clear. In addition, whether IDO controls both immunodominant and subdominant TCD8 is not fully understood. This is an important question because the dominance status of tumor- and virus-specific TCD8 may determine their significance in protective immunity and in vaccine design. We evaluated the magnitude and breadth of cross-primed TCD8 responses to simian virus 40 (SV40) large T antigen as well as primary and recall TCD8 responses to influenza A virus (IAV) in the absence or presence of IDO. IDO(-/-) mice and wild-type mice treated with 1-methyl-D-tryptophan, a pharmacological inhibitor of IDO, exhibited augmented responses to immunodominant epitopes encoded by T antigen and IAV. IDO-mediated suppression of these responses was independent of CD4(+)CD25(+)FoxP3(+) regulatory T cells, which remained numerically and functionally intact in IDO(-/-) mice. Treatment with L-kynurenine failed to inhibit TCD8 responses, indicating that tryptophan metabolites are not responsible for the suppressive effect of IDO in our models. Immunodominant T antigen-specific TCD8 from IDO(-/-) mice showed increased Ki-67 expression, suggesting that they may have acquired a more vigorous proliferative capacity in vivo. In conclusion, IDO suppresses immunodominant TCD8 responses to tumor and viral antigens. Our work also demonstrates that systemic primary and recall TCD8 responses to IAV are controlled by IDO. Inhibition of IDO thus represents an attractive adjuvant strategy in boosting anticancer and antiviral TCD8 targeting highly immunogenic antigens.


