Amyloid β-Protein (1-15)Principal component of amyloid CAS# 183745-81-5 |
- Amyloid β-Peptide (10-20) (human)
Catalog No.:BCC1026
CAS No.:152286-31-2
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
- Myelin Basic Protein (68-82), guinea pig
Catalog No.:BCC1020
CAS No.:98474-59-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 183745-81-5 | SDF | Download SDF |
| PubChem ID | 71464361 | Appearance | Powder |
| Formula | C78H107N25O27 | M.Wt | 1826.86 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >182.6mg/mL in DMSO | ||
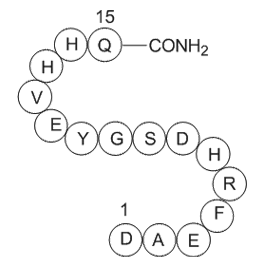
| Sequence | H2N-Asp-Ala-Glu-Phe-Arg-His-Asp-Ser-Gly-Tyr-Glu-Val-His-His-Gln-OH | ||
| Chemical Name | (2S)-5-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-3-carboxypropanoyl]amino]propanoyl]amino]-4-carboxybutanoyl]amino]-3-phenylpropanoyl]amino]-5-carbamimidamidopentanoyl]amino]-3-(1H-imidazol-4-yl)propanoyl]amino]-3-carboxypropanoyl]amino]-3-hydroxypropanoyl]amino]acetyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-carboxybutanoyl]amino]-3-methylbutanoyl]amino]-3-(1H-imidazol-4-yl)propanoyl]amino]-3-(1H-imidazol-4-yl)propanoyl]amino]-5-oxopentanoic acid | ||
| SMILES | CC(C)C(C(=O)NC(CC1=CNC=N1)C(=O)NC(CC2=CNC=N2)C(=O)NC(CCC(=O)N)C(=O)O)NC(=O)C(CCC(=O)O)NC(=O)C(CC3=CC=C(C=C3)O)NC(=O)CNC(=O)C(CO)NC(=O)C(CC(=O)O)NC(=O)C(CC4=CNC=N4)NC(=O)C(CCCNC(=N)N)NC(=O)C(CC5=CC=CC=C5)NC(=O)C(CCC(=O)O)NC(=O)C(C)NC(=O)C(CC(=O)O)N | ||
| Standard InChIKey | CSCAPVBQOYQJJF-NOZGEEMHSA-N | ||
| Standard InChI | InChI=1S/C78H107N25O27/c1-37(2)63(76(128)101-54(26-43-31-85-36-90-43)74(126)99-52(24-41-29-83-34-88-41)72(124)96-49(77(129)130)15-18-57(80)106)103-69(121)48(17-20-60(110)111)95-70(122)50(23-40-11-13-44(105)14-12-40)92-58(107)32-87-66(118)56(33-104)102-75(127)55(28-62(114)115)100-73(125)53(25-42-30-84-35-89-42)98-67(119)46(10-7-21-86-78(81)82)94-71(123)51(22-39-8-5-4-6-9-39)97-68(120)47(16-19-59(108)109)93-64(116)38(3)91-65(117)45(79)27-61(112)113/h4-6,8-9,11-14,29-31,34-38,45-56,63,104-105H,7,10,15-28,32-33,79H2,1-3H3,(H2,80,106)(H,83,88)(H,84,89)(H,85,90)(H,87,118)(H,91,117)(H,92,107)(H,93,116)(H,94,123)(H,95,122)(H,96,124)(H,97,120)(H,98,119)(H,99,126)(H,100,125)(H,101,128)(H,102,127)(H,103,121)(H,108,109)(H,110,111)(H,112,113)(H,114,115)(H,129,130)(H4,81,82,86)/t38-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,55-,56-,63-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Amyloid β-Protein (1-15) Dilution Calculator

Amyloid β-Protein (1-15) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Beta-amyloid protein (A beta), a 39-43 amino acid peptide composed of a portion of the transmembrane domain and the extracellular domain of the amyloid precursor protein (APP), is also the principal component of amyloid. Research found that amyloid beta-protein circulates in human blood and in cerebrospinal fluid and is deposited into plaques found in the brains of patients with Alzheimer's disease (AD). The aggregation of the beta-amyloid protein into dense plaques on the exterior of brain cells results in destroys of the synapses and conduction of nerve impulses.
Ref:
1. Sisodia SS, Price DL. Role of the beta-amyloid protein in Alzheimer's disease. FASEB J. 1995 Mar; 9(5): 366-70.
- MRS 1220
Catalog No.:BCC6972
CAS No.:183721-15-5
- 1,2,3,4,5,6-Hexabromocyclohexane
Catalog No.:BCC2437
CAS No.:1837-91-8
- Penthiopyrad
Catalog No.:BCC8072
CAS No.:183675-82-3
- CYN 154806
Catalog No.:BCC5823
CAS No.:183658-72-2
- Cleroindicin A
Catalog No.:BCC8916
CAS No.:176598-06-4
- Apicidin
Catalog No.:BCC3599
CAS No.:183506-66-3
- CPPG
Catalog No.:BCC6872
CAS No.:183364-82-1
- Erlotinib
Catalog No.:BCC1557
CAS No.:183321-74-6
- OSI-420
Catalog No.:BCC4472
CAS No.:183320-51-6
- Erlotinib Hydrochloride
Catalog No.:BCC3645
CAS No.:183319-69-9
- 5-(1-Piperazinyl)benzofuran-2-carboxamide
Catalog No.:BCC8717
CAS No.:183288-46-2
- 2-Acetoxy-3-deacetoxycaesaldekarin E
Catalog No.:BCN7476
CAS No.:18326-06-2
- Cyanidin 3-sophoroside chloride
Catalog No.:BCN2611
CAS No.:18376-31-3
- Mithramycin A
Catalog No.:BCC2470
CAS No.:18378-89-7
- Ciproxifan
Catalog No.:BCC4539
CAS No.:184025-18-1
- Ciproxifan maleate
Catalog No.:BCC4049
CAS No.:184025-19-2
- sitaxsentan
Catalog No.:BCC1951
CAS No.:184036-34-8
- Dimeric coniferyl acetate
Catalog No.:BCN1148
CAS No.:184046-40-0
- Calystegine B4
Catalog No.:BCN1881
CAS No.:184046-85-3
- Isoleojaponin
Catalog No.:BCN7442
CAS No.:1840966-49-5
- Hautriwaic acid
Catalog No.:BCN4686
CAS No.:18411-75-1
- GB 2a
Catalog No.:BCN7425
CAS No.:18412-96-9
- SR 142948
Catalog No.:BCC7323
CAS No.:184162-64-9
- Dihydromorin
Catalog No.:BCN1149
CAS No.:18422-83-8
The Gut Microbiota and Alzheimer's Disease.[Pubmed:28372330]
J Alzheimers Dis. 2017;58(1):1-15.
The gut microbiota comprises a complex community of microorganism species that resides in our gastrointestinal ecosystem and whose alterations influence not only various gut disorders but also central nervous system disorders such as Alzheimer's disease (AD). AD, the most common form of dementia, is a neurodegenerative disorder associated with impaired cognition and cerebral accumulation of amyloid-beta peptides (Abeta). Most notably, the microbiota-gut-brain axis is a bidirectional communication system that is not fully understood, but includes neural, immune, endocrine, and metabolic pathways. Studies in germ-free animals and in animals exposed to pathogenic microbial infections, antibiotics, probiotics, or fecal microbiota transplantation suggest a role for the gut microbiota in host cognition or AD-related pathogenesis. The increased permeability of the gut and blood-brain barrier induced by microbiota dysbiosis may mediate or affect AD pathogenesis and other neurodegenerative disorders, especially those associated with aging. In addition, bacteria populating the gut microbiota can secrete large amounts of amyloids and lipopolysaccharides, which might contribute to the modulation of signaling pathways and the production of proinflammatory cytokines associated with the pathogenesis of AD. Moreover, imbalances in the gut microbiota can induce inflammation that is associated with the pathogenesis of obesity, type 2 diabetes mellitus, and AD. The purpose of this review is to summarize and discuss the current findings that may elucidate the role of the gut microbiota in the development of AD. Understanding the underlying mechanisms may provide new insights into novel therapeutic strategies for AD.
Potential biomarkers and novel pharmacological targets in protein aggregation-related neurodegenerative diseases.[Pubmed:28159621]
Biochem Pharmacol. 2017 May 1;131:1-15.
The aggregation of specific proteins plays a pivotal role in the etiopathogenesis of several neurodegenerative diseases (NDs). beta-Amyloid (Abeta) peptide-containing plaques and intraneuronal neurofibrillary tangles composed of hyperphosphorylated protein tau are the two main neuropathological lesions in Alzheimer's disease. Meanwhile, Parkinson's disease is defined by the presence of intraneuronal inclusions (Lewy bodies), in which alpha-synuclein (alpha-syn) has been identified as a major protein component. The current literature provides considerable insights into the mechanisms underlying oligomeric-related neurodegeneration, as well as the relationship between protein aggregation and ND, thus facilitating the development of novel putative biomarkers and/or pharmacological targets. Recently, alpha-syn, tau and Abeta have been shown to interact each other or with other "pathological proteins" to form toxic heteroaggregates. These latest findings are overcoming the concept that each neurodegenerative disease is related to the misfolding of a single specific protein. In this review, potential opportunities and pharmacological approaches targeting alpha-syn, tau and Abeta and their oligomeric forms are highlighted with examples from recent studies. Protein aggregation as a biomarker of NDs, in both the brain and peripheral fluids, is deeply explored. Finally, the relationship between biomarker establishment and assessment and their use as diagnostics or therapeutic targets are discussed.
An Anti-beta-Amyloid Vaccine for Treating Cognitive Deficits in a Mouse Model of Down Syndrome.[Pubmed:27023444]
PLoS One. 2016 Mar 29;11(3):e0152471.
In Down syndrome (DS) or trisomy of chromosome 21, the beta-amyloid (Abeta) peptide product of the amyloid precursor protein (APP) is present in excess. Evidence points to increased APP gene dose and Abeta as playing a critical role in cognitive difficulties experienced by people with DS. Particularly, Abeta is linked to the late-life emergence of dementia as associated with neuropathological markers of Alzheimer's disease (AD). At present, no treatment targets Abeta-related pathogenesis in people with DS. Herein we used a vaccine containing the Abeta 1-15 peptide embedded into liposomes together with the adjuvant monophosphoryl lipid A (MPLA). Ts65Dn mice, a model of DS, were immunized with the anti-Abeta vaccine at 5 months of age and were examined for cognitive measures at 8 months of age. The status of basal forebrain cholinergic neurons and brain levels of APP and its proteolytic products were measured. Immunization of Ts65Dn mice resulted in robust anti-Abeta IgG titers, demonstrating the ability of the vaccine to break self-tolerance. The vaccine-induced antibodies reacted with Abeta without detectable binding to either APP or its C-terminal fragments. Vaccination of Ts65Dn mice resulted in a modest, but non-significant reduction in brain Abeta levels relative to vehicle-treated Ts65Dn mice, resulting in similar levels of Abeta as diploid (2N) mice. Importantly, vaccinated Ts65Dn mice showed resolution of memory deficits in the novel object recognition and contextual fear conditioning tests, as well as reduction of cholinergic neuron atrophy. No treatment adverse effects were observed; vaccine did not result in inflammation, cellular infiltration, or hemorrhage. These data are the first to show that an anti-Abeta immunotherapeutic approach may act to target Abeta-related pathology in a mouse model of DS.
Neuroimaging biomarkers and impaired olfaction in cognitively normal individuals.[Pubmed:28543731]
Ann Neurol. 2017 Jun;81(6):871-882.
OBJECTIVE: There is a need for inexpensive noninvasive tests to identify older healthy persons at risk for Alzheimer disease (AD) for enrollment in AD prevention trials. Our objective was to examine whether abnormalities in neuroimaging measures of amyloid and neurodegeneration are correlated with odor identification (OI) in the population-based Mayo Clinic Study of Aging. METHODS: Cognitively normal (CN) participants had olfactory function assessed using the Brief Smell Identification Test (B-SIT), underwent magnetic resonance imaging (n = 829) to assess a composite AD signature cortical thickness and hippocampal volume (HVa), and underwent (11) C-Pittsburgh compound B (n = 306) and (18) fluorodeoxyglucose (n = 305) positron emission tomography scanning to assess amyloid accumulation and brain hypometabolism, respectively. The association of neuroimaging biomarkers with OI was examined using multinomial logistic regression and simple linear regression models adjusted for potential confounders. RESULTS: Among 829 CN participants (mean age = 79.2 years; 51.5% men), 248 (29.9%) were normosmic and 78 (9.4%) had anosmia (B-SIT score < 6). Abnormal AD signature cortical thickness and reduced HVa were associated with decreased OI as a continuous measure (slope = -0.43, 95% confidence interval [CI] = -0.76 to -0.09, p = 0.01 and slope = -0.72, 95% CI = -1.15 to -0.28, p < 0.01, respectively). Reduced HVa, decreased AD signature cortical thickness, and increased amyloid accumulation were significantly associated with increased odds of anosmia. INTERPRETATION: Our findings suggest that OI may be a noninvasive, inexpensive marker for risk stratification, for identifying participants at the preclinical stage of AD who may be at risk for cognitive impairment and eligible for inclusion in AD prevention clinical trials. These cross-sectional findings remain to be validated prospectively. Ann Neurol 2017;81:871-882.
Determination of acidity constants and prediction of electrophoretic separation of amyloid beta peptides.[Pubmed:28619586]
J Chromatogr A. 2017 Jul 28;1508:148-157.
In this paper we describe a strategy to estimate by CE the acidity constants (pKa) of complex polyprotic peptides from their building peptide fragments. CE has been used for the determination of the pKas of five short polyprotic peptides that cover all the sequence of amyloid beta (Abeta) peptides 1-40 and 1-42 (Abeta fragments 1-15, 10-20, 20-29, 25-35 and 33-42). First, the electrophoretic mobility (me) was measured as a function of pH of the background electrolyte (BGE) in the pH range 2-12 (bare fused silica capillary, I=25mM and T=25 degrees C). Second, the mes were fitted to equations modelling the ionisable behaviour of the different fragments as a function of pH to determine their pKas. The accuracy of the pKas was demonstrated predicting the electrophoretic behaviour of the studied fragments using the classical semiempirical relationships between me and peptide charge-to-mass ratio (me vs. q/Mr(1/2), classical polymer model, q=charge and Mr=relative molecular mass). Separation selectivity in a mixture of the fragments as a function of pH was evaluated, taking into account the influence of the electroosmotic flow (EOF) at each pH value, and a method for the simple and rapid simulation of the electropherograms at the optimum separation pH was described. Finally, the pKas of the fragments were used to estimate the pKas of the Abeta peptides 1-40 and 1-42 ((t)C and D 3.1, E 4.6 and Y 10.8 for acidic amino acids and (t)N-D 8.6, H 6.0, K 10.6 and R 12.5 for basic amino acids), which were used to predict their behaviour and simulate their electropherograms with excellent results. However, as expected due to the very small differences on q/Mr(1/2) values, separation resolution of their mixtures was poor over the whole pH range. The use of poly(vinyl alcohol) (PVA) coated capillaries allowed reducing the EOF and a slight improvement of resolution.


