15-Isopimarene-8,18-diolCAS# 73002-86-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 73002-86-5 | SDF | Download SDF |
| PubChem ID | 101465555 | Appearance | Powder |
| Formula | C20H34O2 | M.Wt | 306.5 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Synonyms | 8-Hydroxysandaracopimarinol | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
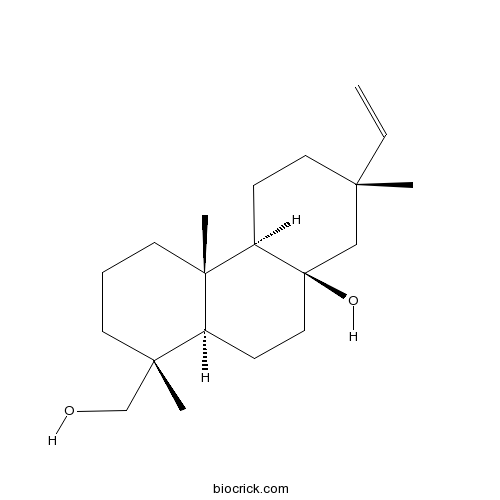
| Chemical Name | (1R,4aS,4bR,7S,8aR,10aR)-7-ethenyl-1-(hydroxymethyl)-1,4a,7-trimethyl-2,3,4,4b,5,6,8,9,10,10a-decahydrophenanthren-8a-ol | ||
| SMILES | CC1(CCC2C3(CCCC(C3CCC2(C1)O)(C)CO)C)C=C | ||
| Standard InChIKey | RIQATFSOVFFVRX-DWIKVQACSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

15-Isopimarene-8,18-diol Dilution Calculator

15-Isopimarene-8,18-diol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2626 mL | 16.3132 mL | 32.6264 mL | 65.2529 mL | 81.5661 mL |
| 5 mM | 0.6525 mL | 3.2626 mL | 6.5253 mL | 13.0506 mL | 16.3132 mL |
| 10 mM | 0.3263 mL | 1.6313 mL | 3.2626 mL | 6.5253 mL | 8.1566 mL |
| 50 mM | 0.0653 mL | 0.3263 mL | 0.6525 mL | 1.3051 mL | 1.6313 mL |
| 100 mM | 0.0326 mL | 0.1631 mL | 0.3263 mL | 0.6525 mL | 0.8157 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lidocaine hydrochloride
Catalog No.:BCC9009
CAS No.:73-78-9
- Guanine
Catalog No.:BCN8414
CAS No.:73-40-5
- H-Ile-OH
Catalog No.:BCC2960
CAS No.:73-32-5
- Melatonin
Catalog No.:BCN2196
CAS No.:73-31-4
- Adenine
Catalog No.:BCC4450
CAS No.:73-24-5
- H-Trp-OH
Catalog No.:BCC3111
CAS No.:73-22-3
- Cordycepin
Catalog No.:BCN5389
CAS No.:73-03-0
- L-(-)-threo-3-Hydroxyaspartic acid
Catalog No.:BCC6565
CAS No.:7298-99-9
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
- BMS-707035
Catalog No.:BCC2133
CAS No.:729607-74-3
- XRP44X
Catalog No.:BCC7568
CAS No.:729605-21-4
- Gomisin O
Catalog No.:BCN2875
CAS No.:72960-22-6
- CyPPA
Catalog No.:BCC7526
CAS No.:73029-73-9
- Atractylenolide III
Catalog No.:BCN1045
CAS No.:73030-71-4
- Epigomisin O
Catalog No.:BCN2862
CAS No.:73036-31-4
- Atractylenolide I
Catalog No.:BCN1043
CAS No.:73069-13-3
- Atractylenolide II
Catalog No.:BCN1044
CAS No.:73069-14-4
- Praeruptorin A
Catalog No.:BCN4987
CAS No.:73069-25-7
- (+)-Praeruptorin A
Catalog No.:BCN4989
CAS No.:73069-27-9
- Praeruptorin D
Catalog No.:BCN4990
CAS No.:73069-28-0
- Scutebarbatine D
Catalog No.:BCN8536
CAS No.:910099-76-2
- Arecoline
Catalog No.:BCN8537
CAS No.:73069-28-9
- Ferruginine
Catalog No.:BCN1911
CAS No.:73069-63-3
- Fenticonazole nitrate
Catalog No.:BCC8983
CAS No.:73151-29-8
Active principles of Tetradenia riparia. I. Antimicrobial activity of 8(14),15-sandaracopimaradiene-7 alpha,18-diol.[Pubmed:3807389]
J Ethnopharmacol. 1986 Sep;17(3):269-75.
Tetradenia riparia is one of the most popular medicinal plants in Rwanda. Previously, several new substances have been isolated from the leaves of this plant, including a new diterpene diol, i.e. 8(14),15-sandaracopimaradiene-7 alpha,18-diol. This new diterpene diol exhibits significant antimicrobial activity against several bacteria and fungi. The minimum inhibitory concentration (MIC) of the substance for microorganisms which were inhibited ranged from 6.25 to 100 micrograms/ml.
Acute and neurotoxicity of two structurally related acetylenic compounds: 5,7,11-dodecatriyn-1-ol and 5,7,11,13-octadecatetrayne-1,18-diol.[Pubmed:3356863]
J Appl Toxicol. 1988 Feb;8(1):35-42.
Two structurally related acetylenic compounds, 5,7,11-Dodecatriyn-1-ol, (Compound A), and 5,7,11,13-Octadecatetrayne-1,18-Diol (Compound B), were evaluated in a tier I toxicology testing program as part of an ongoing research and development program. This battery of acute tests included acute oral, guinea pig maximization, photosensitization, dermal irritation, Ames and multiple genetic endpoint and a 2 week oral fetotoxicity study. Compound A was found to have an oral LD50 of 0.25 ml/kg, be an extreme dermal sensitizer, a mild dermal irritant (PDII of 1.7), and not mutagenic or fetotoxic in the tests employed. Compound B had an oral LD50 greater than 4 g/kg, was a moderate dermal sensitizer and mild dermal irritant (PDII of 1.4), was not mutagenic in the Ames test but weakly increased the incidence of SCEs and gene mutations in Chinese Hamster Ovary cells, and was not fetotoxic. Neither compound was found to be a photosensitizer, but during the course of the photosensitization study Compound A was found to cause neuromuscular signs (including hind limb paralysis) and a bilateral necrosis of the medulla oblongata in female guinea pigs. A similar lesion was found in female rats receiving a single oral dose of 0.25 ml/kg and in nonpregnant females dosed daily for two weeks at 0.03 ml/kg. Compound B was not found to produce any of these neurologic effects.
ent-5alpha,3,15-Dioxodolabr-4(18)-ene-16,18-diol.[Pubmed:21589075]
Acta Crystallogr Sect E Struct Rep Online. 2010 Oct 23;66(Pt 11):o2897-8.
The title compound, C(20)H(30)O(4), is a dolabrane diterpenoid isolated from Ceriops tagal, in which one of the three fused cyclo-hexane rings adopts a half-chair conformation and the other two are in the standard chair conformations. The hy-droxy-methyl-idene substituent is attached to the half-chair cyclo-hexane. An intra-molecular O-Hcdots, three dots, centeredO hydrogen bond generate an S(6) ring motif. In the crystal, mol-ecules are arranged into screw chains along the [001] direction. The crystal is stabilized by O-Hcdots, three dots, centeredO hydrogen bonds and weaker C-Hcdots, three dots, centeredO inter-actions.


