Jasminoside BCAS# 214125-04-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 214125-04-9 | SDF | Download SDF |
| PubChem ID | 102507168 | Appearance | Powder |
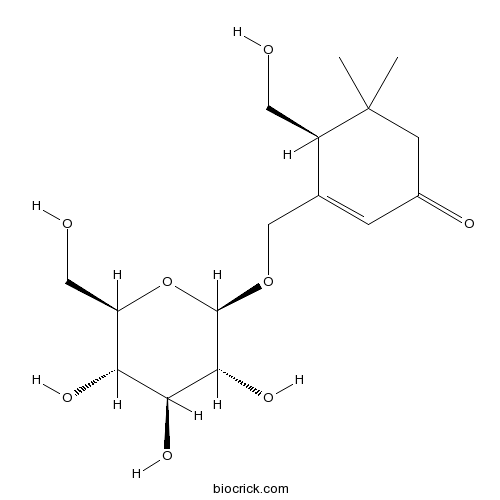
| Formula | C16H26O8 | M.Wt | 346.37 |
| Type of Compound | Monoterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (4S)-4-(hydroxymethyl)-5,5-dimethyl-3-[[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]cyclohex-2-en-1-one | ||
| SMILES | CC1(CC(=O)C=C(C1CO)COC2C(C(C(C(O2)CO)O)O)O)C | ||
| Standard InChIKey | XBRXLXOCHNGHBC-PLJUSGQGSA-N | ||
| Standard InChI | InChI=1S/C16H26O8/c1-16(2)4-9(19)3-8(10(16)5-17)7-23-15-14(22)13(21)12(20)11(6-18)24-15/h3,10-15,17-18,20-22H,4-7H2,1-2H3/t10-,11-,12-,13+,14-,15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Chinese Traditional & Herbal Drugs, 2013, 44(13):1730-1733.Chemical constituents of monoterpenes in fruits of Gardenia jasminoides.[Reference: WebLink]
To investigate the chemical constituents of monoterpenes in the fruits of Gardenia jasminoides. |

Jasminoside B Dilution Calculator

Jasminoside B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8871 mL | 14.4354 mL | 28.8709 mL | 57.7417 mL | 72.1772 mL |
| 5 mM | 0.5774 mL | 2.8871 mL | 5.7742 mL | 11.5483 mL | 14.4354 mL |
| 10 mM | 0.2887 mL | 1.4435 mL | 2.8871 mL | 5.7742 mL | 7.2177 mL |
| 50 mM | 0.0577 mL | 0.2887 mL | 0.5774 mL | 1.1548 mL | 1.4435 mL |
| 100 mM | 0.0289 mL | 0.1444 mL | 0.2887 mL | 0.5774 mL | 0.7218 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cinobufaginol
Catalog No.:BCN8593
CAS No.:6691-83-4
- Licoflavone B
Catalog No.:BCN8592
CAS No.:91433-17-9
- Firmanoic acid
Catalog No.:BCN8591
CAS No.:107584-83-8
- Aaptamine
Catalog No.:BCN8590
CAS No.:85547-22-4
- Hydroquinidine
Catalog No.:BCN8589
CAS No.:1435-55-8
- Rubiayannone A
Catalog No.:BCN8588
CAS No.:517978-25-1
- Glyasperin C
Catalog No.:BCN8587
CAS No.:142474-53-1
- Sarracenin
Catalog No.:BCN8586
CAS No.:59653-37-1
- Platycodin D3
Catalog No.:BCN8585
CAS No.:67884-03-1
- Lucidin 3-O-primeveroside
Catalog No.:BCN8584
CAS No.:29706-59-0
- Ligupurpuroside C
Catalog No.:BCN8583
CAS No.:1194056-33-1
- Isorosmanol
Catalog No.:BCN8582
CAS No.:93780-80-4
- Ophiopogonoside A
Catalog No.:BCN8595
CAS No.:791849-22-4
- Dehydronuciferine
Catalog No.:BCN8596
CAS No.:7630-74-2
- Licoflavone A
Catalog No.:BCN8597
CAS No.:61153-77-3
- Eriosematin
Catalog No.:BCN8598
CAS No.:168010-17-1
- Isoastragaloside IV
Catalog No.:BCN8599
CAS No.:136033-55-1
- Astraganoside
Catalog No.:BCN8600
CAS No.:1011711-05-9
- Rubipodanone A
Catalog No.:BCN8601
CAS No.:2170211-22-8
- Asperosaponin IV
Catalog No.:BCN8602
CAS No.:126778-93-6
- 9-Methymyrrhone
Catalog No.:BCN8603
CAS No.:1809980-22-0
- Uvarigranol C
Catalog No.:BCN8604
CAS No.:172104-04-0
- Saikosaponin I
Catalog No.:BCN8605
CAS No.:103629-71-6
- Toringin
Catalog No.:BCN8606
CAS No.:1329-10-8
A two-step ultra-high-performance liquid chromatography-quadrupole/time of flight mass spectrometry with mass defect filtering method for rapid identification of analogues from known components of different chemical structure types in Fructus Gardeniae-Fructus Forsythiae herb pair extract and in rat's blood.[Pubmed:29861306]
J Chromatogr A. 2018 Aug 17;1563:99-123.
Fructus Gardeniae-Fructus Forsythiae herb pair is an herbal formula used extensively to treat inflammation and fever, but few systematic identification studies of the bioactive components have been reported. Herein, the unknown analogues in the first-step screening were rapidly identified from representative compounds in different structure types (geniposide as iridoid type, crocetin as crocetin type, Jasminoside B as monocyclic monoterpene type, oleanolic acid as saponin type, 3-caffeoylquinic acid as organic acid type, forsythoside A as phenylethanoid type, phillyrin as lignan type and quercetin 3-rutinoside as flavonoid type) by UPLC-Q-Tof/MS combined with mass defect filtering (MDF), and further confirmed with reference standards and published literatures. Similarly, in the second step, other unknown components were rapidly discovered from the compounds identified in the first step by MDF. Using the two-step screening method, a total of 58 components were characterized in Fructus Gardeniae-Fructus Forsythiae (FG-FF) decoction. In rat's blood, 36 compounds in extract and 16 metabolites were unambiguously or tentatively identified. Besides, we found the principal metabolites were glucuronide conjugates, with the glucuronide conjugates of caffeic acid, quercetin and kaempferol confirmed as caffeic acid 3-glucuronide, quercetin 3-glucuronide and kaempferol 3-glucuronide by reference standards, respectively. Additionally, most of them bound more strongly to human serum albumin than their respective prototypes, predicted by Molecular Docking and Simulation, indicating that they had lower blood clearance in vivo and possibly more contribution to pharmacological effects. This study developed a novel two-step screening method in addressing how to comprehensively screen components in herbal medicine by UPLC-Q-Tof/MS with MDF.


