Glyasperin CCAS# 142474-53-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 142474-53-1 | SDF | Download SDF |
| PubChem ID | 480859 | Appearance | Powder |
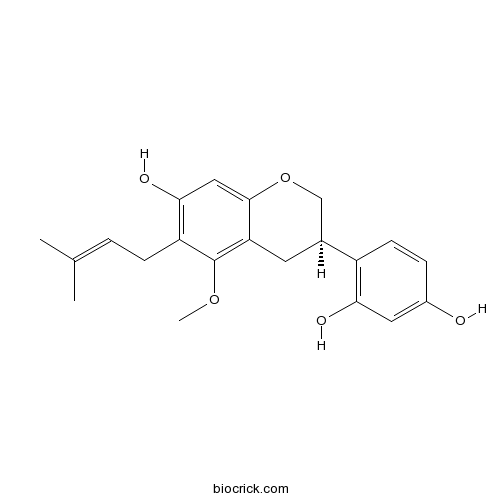
| Formula | C21H24O5 | M.Wt | 356.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-[(3R)-7-hydroxy-5-methoxy-6-(3-methylbut-2-enyl)-3,4-dihydro-2H-chromen-3-yl]benzene-1,3-diol | ||
| SMILES | CC(=CCC1=C(C=C2C(=C1OC)CC(CO2)C3=C(C=C(C=C3)O)O)O)C | ||
| Standard InChIKey | RCZMWVKBVFOCEE-ZDUSSCGKSA-N | ||
| Standard InChI | InChI=1S/C21H24O5/c1-12(2)4-6-16-19(24)10-20-17(21(16)25-3)8-13(11-26-20)15-7-5-14(22)9-18(15)23/h4-5,7,9-10,13,22-24H,6,8,11H2,1-3H3/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Glyasperin C acts as a partial estrogen antagonist. 2. Glyasperin C shows potent anti-vancomycin-resistant Enterococci effects (MIC 1.9 × 10-5-4.5 × 10-5 M for E. faecium and E. faecalis). 3. Glyasperin C shows tyrosinase inhibitory activity (IC (50) = 0.13 +/- 0.01 microg/mL), it could be a promising candidate in the design of skin-whitening agents. |
| Targets | Estrogen receptor | Tyrosinase | Antifection | Progestogen receptor |

Glyasperin C Dilution Calculator

Glyasperin C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8058 mL | 14.0292 mL | 28.0584 mL | 56.1167 mL | 70.1459 mL |
| 5 mM | 0.5612 mL | 2.8058 mL | 5.6117 mL | 11.2233 mL | 14.0292 mL |
| 10 mM | 0.2806 mL | 1.4029 mL | 2.8058 mL | 5.6117 mL | 7.0146 mL |
| 50 mM | 0.0561 mL | 0.2806 mL | 0.5612 mL | 1.1223 mL | 1.4029 mL |
| 100 mM | 0.0281 mL | 0.1403 mL | 0.2806 mL | 0.5612 mL | 0.7015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sarracenin
Catalog No.:BCN8586
CAS No.:59653-37-1
- Platycodin D3
Catalog No.:BCN8585
CAS No.:67884-03-1
- Lucidin 3-O-primeveroside
Catalog No.:BCN8584
CAS No.:29706-59-0
- Ligupurpuroside C
Catalog No.:BCN8583
CAS No.:1194056-33-1
- Isorosmanol
Catalog No.:BCN8582
CAS No.:93780-80-4
- Isoginsenoside Rh3
Catalog No.:BCN8581
CAS No.:166040-90-0
- Uvarigrin
Catalog No.:BCN8580
CAS No.:200563-11-7
- 4-Benzamido-2,5-diethoxybenzenediazonium
Catalog No.:BCN8579
CAS No.:5486-84-0
- Hexamethylquercetagetin
Catalog No.:BCN8578
CAS No.:1251-84-9
- Epitheaflagallin 3-O-gallate
Catalog No.:BCN8577
CAS No.:102067-92-5
- 5,6-Dehydroginsenoside Rd
Catalog No.:BCN8576
CAS No.:1268459-68-2
- Jionoside D
Catalog No.:BCN8575
CAS No.:120406-34-0
- Rubiayannone A
Catalog No.:BCN8588
CAS No.:517978-25-1
- Hydroquinidine
Catalog No.:BCN8589
CAS No.:1435-55-8
- Aaptamine
Catalog No.:BCN8590
CAS No.:85547-22-4
- Firmanoic acid
Catalog No.:BCN8591
CAS No.:107584-83-8
- Licoflavone B
Catalog No.:BCN8592
CAS No.:91433-17-9
- Cinobufaginol
Catalog No.:BCN8593
CAS No.:6691-83-4
- Jasminoside B
Catalog No.:BCN8594
CAS No.:214125-04-9
- Ophiopogonoside A
Catalog No.:BCN8595
CAS No.:791849-22-4
- Dehydronuciferine
Catalog No.:BCN8596
CAS No.:7630-74-2
- Licoflavone A
Catalog No.:BCN8597
CAS No.:61153-77-3
- Eriosematin
Catalog No.:BCN8598
CAS No.:168010-17-1
- Isoastragaloside IV
Catalog No.:BCN8599
CAS No.:136033-55-1
Structures of new phenolics isolated from licorice, and the effectiveness of licorice phenolics on vancomycin-resistant Enterococci.[Pubmed:25157467]
Molecules. 2014 Aug 25;19(9):13027-41.
Licorice, which is the underground part of Glycyrrhiza species, has been used widely in Asian and Western countries as a traditional medicine and as a food additive. Our continuous investigation on the constituents of roots and stolons of Glycyrrhiza uralensis led to the isolation of two new phenolics, in addition to 14 known compounds. Structural studies including spectroscopic and simple chemical derivatizations revealed that both of the new compounds had 2-aryl-3-methylbenzofuran structures. An examination of the effectiveness of licorice phenolics obtained in this study on vancomycin-resistant strains Enterococcus faecium FN-1 and Enterococcus faecalis NCTC12201 revealed that licoricidin showed the most potent antibacterial effects against both of E. faecalis and E. faecium with a minimum inhibitory concentration (MIC) of 1.9 x 10-5 M. 8-(gamma,gamma-Dimethylallyl)-wighteone, isoangustone A, 3'-(gamma,gamma-dimethylallyl)-kievitone, Glyasperin C, and one of the new 3-methyl-2-phenylbenzofuran named neoglycybenzofuran also showed potent anti-vancomycin-resistant Enterococci effects (MIC 1.9 x 10-5-4.5 x 10-5 M for E. faecium and E. faecalis). The HPLC condition for simultaneous detection of the phenolics in the extract was investigated to assess the quality control of the natural antibacterial resource, and quantitative estimation of several major phenolics in the extract with the established HPLC condition was also performed. The results showed individual contents of 0.08%-0.57% w/w of EtOAc extract for the major phenolics in the materials examined.
Identification of tyrosinase inhibitors from Glycyrrhiza uralensis.[Pubmed:16142649]
Planta Med. 2005 Aug;71(8):785-7.
Tyrosinase is a key enzyme in the production of melanins. Phytochemical studies of a Glycyrrhiza uralensis extract were performed by measuring the tyrosinase and melanin synthesis inhibitory activity. Glycyrrhisoflavone and Glyasperin C were identified as tyrosinase inhibitors for the first time. Glyasperin C showed a stronger tyrosinase inhibitory activity (IC (50) = 0.13 +/- 0.01 microg/mL) than glabridin (IC (50) = 0.25 +/- 0.01 microg/mL) and a moderate inhibition of melanin production (17.65 +/- 8.8 % at 5 microg/mL). Glycyrrhisoflavone showed a strong melanin synthesis inhibitory activity (63.73 +/- 6.8 % inhibition at 5 microg/mL). These results suggest that Glyasperin C and glycyrrhisoflavone could be promising candidates in the design of skin-whitening agents.
Licorice root components in dietary supplements are selective estrogen receptor modulators with a spectrum of estrogenic and anti-estrogenic activities.[Pubmed:26631549]
Steroids. 2016 Jan;105:42-9.
Licorice root extracts are often consumed as botanical dietary supplements by menopausal women as a natural alternative to pharmaceutical hormone replacement therapy. In addition to their components liquiritigenin (Liq) and isoliquiritigenin (Iso-Liq), known to have estrogenic activity, licorice root extracts also contain a number of other flavonoids, isoflavonoids, and chalcones. We have investigated the estrogenic activity of 7 of these components, obtained from an extract of Glycyrrhiza glabra powder, namely Glabridin (L1), Calycosin (L2), Methoxychalcone (L3), Vestitol (L4), Glyasperin C (L5), Glycycoumarin (L6), and Glicoricone (L7), and compared them with Liq, Iso-Liq, and estradiol (E2). All components, including Liq and Iso-Liq, have low binding affinity for estrogen receptors (ERs). Their potency and efficacy in stimulating the expression of estrogen-regulated genes reveal that Liq and Iso-Liq and L2, L3, L4, and L6 are estrogen agonists. Interestingly, L3 and L4 have an efficacy nearly equivalent to E2 but with a potency ca. 10,000-fold less. The other components, L1, L5 and L7, acted as partial estrogen antagonists. All agonist activities were reversed by the antiestrogen, ICI 182,780, or by knockdown of ERalpha with siRNA, indicating that they are ER dependent. In HepG2 hepatoma cells stably expressing ERalpha, only Liq, Iso-Liq, and L3 stimulated estrogen-regulated gene expression, and in all cases gene stimulation did not occur in HepG2 cells lacking ERalpha. Collectively, these findings classify the components of licorice root extracts as low potency, mixed ER agonists and antagonists, having a character akin to that of selective estrogen receptor modulators or SERMs.


