IsoacteosideCAS# 61303-13-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 61303-13-7 | SDF | Download SDF |
| PubChem ID | 6476333 | Appearance | Powder |
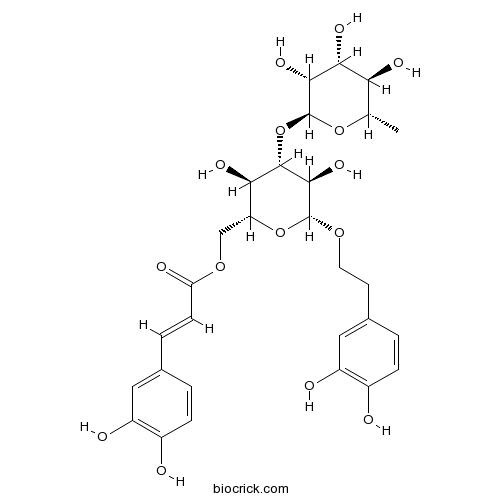
| Formula | C29H36O15 | M.Wt | 624.6 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Synonyms | Isoverbascoside | ||
| Solubility | DMSO : ≥ 48 mg/mL (76.85 mM) | ||
| Chemical Name | [(2R,3R,4S,5R,6R)-6-[2-(3,4-dihydroxyphenyl)ethoxy]-3,5-dihydroxy-4-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]methyl (E)-3-(3,4-dihydroxyphenyl)prop-2-enoate | ||
| SMILES | CC1C(C(C(C(O1)OC2C(C(OC(C2O)OCCC3=CC(=C(C=C3)O)O)COC(=O)C=CC4=CC(=C(C=C4)O)O)O)O)O)O | ||
| Standard InChIKey | FNMHEHXNBNCPCI-QEOJJFGVSA-N | ||
| Standard InChI | InChI=1S/C29H36O15/c1-13-22(35)24(37)25(38)29(42-13)44-27-23(36)20(12-41-21(34)7-4-14-2-5-16(30)18(32)10-14)43-28(26(27)39)40-9-8-15-3-6-17(31)19(33)11-15/h2-7,10-11,13,20,22-33,35-39H,8-9,12H2,1H3/b7-4+/t13-,20+,22-,23+,24+,25+,26+,27-,28+,29-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Isoacteoside exhibits significant inhibition of advanced glycation end product formation with IC50 values of 4.6-25.7 μM; it has neuroprotective, antioxidant, and anti-inflammatory effects. Isoacteoside can stimulate the increase of α7 and α3 proteins in the cultured cells, attenuate the decreased expression of α3 and α7 nAChR subunit proteins and cell viability on SH-SY5Y cells induced by Aβ; it can regulate caspase-1, mitogen-activated protein kinases (c-Jun N-terminal kinase, p38, extracellular signal-regulated protein kinase) and nuclear factor-kappa B pathways. |
| Targets | IL Receptor | TNF-α | Caspase | p38MAPK | NF-kB | ERK | JNK | Beta Amyloid | AChR |
| In vitro | Anti-inflammatory effects of isoacteoside from Abeliophyllum distichum.[Pubmed: 25975581]Immunopharmacol Immunotoxicol. 2015 May 15:1-7.Isoacteoside, a dihydroxypheynylethyl glycoside, is a major bioactive component of Abeliophyllum distichum (White Forsythia) which is a deciduous shrub native to the south and central areas of Korea.
The present study is designed to evaluate the anti-inflammatory activities and underlying mechanisms of Isoacteoside in human mast cell line, HMC-1 cells.
Inhibitory activities of acteoside, isoacteoside, and its structural constituents against protein glycation in vitro.[Pubmed: 28510849 ]Bot Stud. 2013 Dec;54(1):6.Advanced glycation end products (AGE) are substances that can induce insulin resistance in adipocyte, hepatocyte and muscle cells. This resistance correlates highly with cardiovascular disease and diabetic complications. Acteoside (A), a phenylethanoid glycoside, is an active compound in several plants and traditional herbal medicines. Acteoside, its structural isomer, Isoacteoside (I), and their constituents, caffeic acid (C) and 3,4-dihydroxyphenylethanol (D), were used in the study to investigate the inhibitory activity against AGE formations in vitro.
|
| Cell Research | Effects of Echinacoside and Isoacteoside on the Expression of Nicotinic Receptors in Neuroblastoma Cells.[Reference: WebLink]Antioxidant activity of isoacteoside from Clerodendron trichotomum.[Pubmed: 15799629]J Toxicol Environ Health A. 2005 Mar 12;68(5):389-400.The antioxidant properties of Isoacteoside, isolated from Clerodendron trichotomum (Verbenaceae), were investigated.
Lishizhen Medicine & Materia Medica Research, 2011, 22(7):1561-3.To investigate the effects of echinacoside and Isoacteoside on the expression of nicotinic receptor in neuroblastoma SH-SY5Y cells and the protective mechanism against neurotoxicity induced by beta-amyloid peptide(Aβ). |

Isoacteoside Dilution Calculator

Isoacteoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.601 mL | 8.0051 mL | 16.0102 mL | 32.0205 mL | 40.0256 mL |
| 5 mM | 0.3202 mL | 1.601 mL | 3.202 mL | 6.4041 mL | 8.0051 mL |
| 10 mM | 0.1601 mL | 0.8005 mL | 1.601 mL | 3.202 mL | 4.0026 mL |
| 50 mM | 0.032 mL | 0.1601 mL | 0.3202 mL | 0.6404 mL | 0.8005 mL |
| 100 mM | 0.016 mL | 0.0801 mL | 0.1601 mL | 0.3202 mL | 0.4003 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Isoacteoside is a natural compound which exhibit significant inhibition of advanced glycation end product formation with IC50 values of 4.6-25.7 μM, compared with those of aminoguanidine (IC50=1,056 μM) and quercetin (IC50=28.4 μM) as positive controls. IC50 value: Target: In the rat lens aldose reductase assay, acteoside, isoacteoside, and poliumoside exhibited greater inhibitory effects on rat lens aldose reductase with IC50 values of 0.83, 0.83, and 0.85 μM, respectively, than those of the positive controls, 3,3-tetramethyleneglutaric acid (IC50=4.03 μM) and quercetin (IC50=7.2 μM).
References:
[1]. Yu SY, et al. Caffeoylated phenylpropanoid glycosides from Brandisia hancei inhibit advanced glycation end product formation and aldose reductase in vitro and vessel dilation in larval zebrafish in vivo. Planta Med. 2013 Dec;79(18):1705-9.
- Schisandrin C
Catalog No.:BCN1198
CAS No.:61301-33-5
- 2-Aminoacetophenone
Catalog No.:BCC8546
CAS No.:613-89-8
- 2-(Phenylmethoxy)-naphthalene
Catalog No.:BCC8485
CAS No.:613-62-7
- AKT inhibitor VIII
Catalog No.:BCC1334
CAS No.:612847-09-3
- Schizandrin A
Catalog No.:BCN1021
CAS No.:61281-38-7
- Schizandrin B
Catalog No.:BCN1022
CAS No.:61281-37-6
- Boc-D-Phe(4-NO2)-OH
Catalog No.:BCC3276
CAS No.:61280-75-9
- Acteoside
Catalog No.:BCN4136
CAS No.:61276-17-3
- Vitexilactone
Catalog No.:BCN4135
CAS No.:61263-49-8
- Cannabispiran
Catalog No.:BCN4134
CAS No.:61262-81-5
- PTP1B-IN-1
Catalog No.:BCC5506
CAS No.:612530-44-6
- AZD1080
Catalog No.:BCC4508
CAS No.:612487-72-6
- Boc-D-Arg(Tos)-OH
Catalog No.:BCC3070
CAS No.:61315-61-5
- Sulconazole Nitrate
Catalog No.:BCC4853
CAS No.:61318-91-0
- Neoschaftoside
Catalog No.:BCN3053
CAS No.:61328-41-4
- Amoxicillin trihydrate
Catalog No.:BCC5168
CAS No.:61336-70-7
- Boc-D-Gln-OH
Catalog No.:BCC2607
CAS No.:61348-28-5
- Boc-D-Glu-OH
Catalog No.:BCC2606
CAS No.:61348-28-6
- VU 0364770
Catalog No.:BCC4597
CAS No.:61350-00-3
- TNP-ATP triethylammonium salt
Catalog No.:BCC7373
CAS No.:61368-63-6
- erythro-Guaiacylglycerol-beta-O-4'-dehydrodisinapyl ether
Catalog No.:BCN7024
CAS No.:613684-55-2
- Griffonilide
Catalog No.:BCN1271
CAS No.:61371-55-9
- Rifapentine
Catalog No.:BCC4937
CAS No.:61379-65-5
- Trigonelline hydrochloride
Catalog No.:BCN1050
CAS No.:6138-41-6
Antioxidant activity of isoacteoside from Clerodendron trichotomum.[Pubmed:15799629]
J Toxicol Environ Health A. 2005 Mar 12;68(5):389-400.
The antioxidant properties of Isoacteoside, isolated from Clerodendron trichotomum (Verbenaceae), were investigated. This compound scavenged intracellular reactive oxygen species (ROS) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, and prevented lipid peroxidation. This radical scavenging activity of Isoacteoside protected cell viability of Chinese hamster lung fibroblast (V79-4) cells exposed to hydrogen peroxide (H2O2). Furthermore, Isoacteoside reduced the apoptotic cells formation induced by H2O2, as demonstrated by the decreased number of sub-G1 hypo-diploid cells and apoptotic cell body formation. However, Isoacteoside increased the activities of cellular antioxidant enzymes, superoxide dismutase (SOD) and catalase (CAT). Taken together, these findings suggest that Isoacteoside, isolated from C. trichotomum, possesses antioxidant properties.
Anti-inflammatory effects of isoacteoside from Abeliophyllum distichum.[Pubmed:25975581]
Immunopharmacol Immunotoxicol. 2015 Jun;37(3):258-64.
Isoacteoside, a dihydroxypheynylethyl glycoside, is a major bioactive component of Abeliophyllum distichum (White Forsythia) which is a deciduous shrub native to the south and central areas of Korea. The present study is designed to evaluate the anti-inflammatory activities and underlying mechanisms of Isoacteoside in human mast cell line, HMC-1 cells. We isolated Isoacteoside from A. distichum. The anti-inflammatory effect of Isoacteoside was investigated in HMC-1 cells by studying the following markers: phorbol 12-myristate 13-acetate and calcium ionophore A23187 (PMACI)-induced interleukin (IL)-1beta, IL-6, IL-8, and tumor necrosis factor alpha (TNF-alpha) secretion and mRNA expression by ELISA and RT-PCR, respectively. In addition, mechanism related to anti-inflammatory was investigated by Western blotting. Isoacteoside significantly suppressed the production and mRNA expression of proinflammatory cytokines including IL-1beta, IL-6, IL-8 and TNF-alpha in PMACI-stimulated HMC-1 cells without cytotoxicity. It was found that anti-inflammatory effects of Isoacteoside are mediated by action on caspase-1, mitogen-activated protein kinases (c-Jun N-terminal kinase, p38, extracellular signal-regulated protein kinase) and nuclear factor-kappa B pathways. Taken together, the present findings provide new insights that Isoacteoside may be a promising anti-inflammatory agent for inflammatory disorders.
Inhibitory activities of acteoside, isoacteoside, and its structural constituents against protein glycation in vitro.[Pubmed:28510849]
Bot Stud. 2013 Dec;54(1):6.
BACKGROUND: Advanced glycation end products (AGE) are substances that can induce insulin resistance in adipocyte, hepatocyte and muscle cells. This resistance correlates highly with cardiovascular disease and diabetic complications. Acteoside (A), a phenylethanoid glycoside, is an active compound in several plants and traditional herbal medicines. Acteoside, its structural isomer, Isoacteoside (I), and their constituents, caffeic acid (C) and 3,4-dihydroxyphenylethanol (D), were used in the study to investigate the inhibitory activity against AGE formations in vitro. RESULTS: AGE formations were detected by anti-(N()-(carboxymethyl)lysine (anti-CML), using bovine serum albumin (BSA)/glucose (glc) and BSA/galactose (gal) as models, or by anti-argpyrimidine (anti-AP), using BSA/methylglyoxal (MGO) as models. It was found that A, I, C, or D, each at 5 mM, could attenuate the CML formations detected by ELISA in the BSA/gal model of a 3-day or 5-day reaction, and showed significant differences (P < 0.01 or P < 0.001) compared to the control. However, these compounds showed a minor effect after a 7-day incubation. It was also found that C or D could lower the CML formations in the BSA/glc model and showed significant differences (P < 0.05 or P < 0.01) compared to the control after a 3-day, 5-day and 7-day reaction. It was found that A, I, C, or D, each at 0.5 mM or 5 mM, could attenuate the AP formations in the BSA/MGO model of a 3-day reaction and showed significant differences (P < 0.001) compared to the control. CONCLUSIONS: The results suggest the potential anti-glycation activities of A and I in vitro may apply to cell models at higher glucose concentrations or to diabetic animal models, and need further investigation.


