Trigonelline hydrochlorideCAS# 6138-41-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

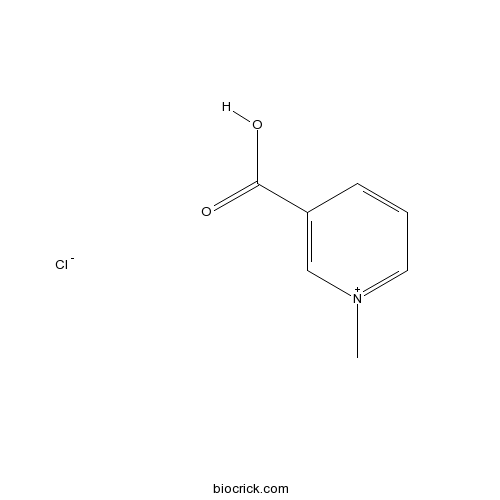
| Cas No. | 6138-41-6 | SDF | Download SDF |
| PubChem ID | 134606 | Appearance | White powder |
| Formula | C7H8ClNO2 | M.Wt | 173.60 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | DMSO : 6 mg/mL (34.56 mM; Need ultrasonic) | ||
| Chemical Name | 1-methylpyridin-1-ium-3-carboxylic acid;chloride | ||
| SMILES | C[N+]1=CC=CC(=C1)C(=O)O.[Cl-] | ||
| Standard InChIKey | TZSYLWAXZMNUJB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H7NO2.ClH/c1-8-4-2-3-6(5-8)7(9)10;/h2-5H,1H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Trigonelline chloride, an alkaloid with potential antidiabetic activity, is present in considerable amounts in coffee. Trigonelline hydrochloride reduces diabetic auditory neuropathy by affecting β cell regeneration. |
| Targets | Beta Amyloid |
| In vivo | Effects of coffee components on the response of GABA(A) receptors expressed in Xenopus oocytes.[Pubmed: 14664509]J Agric Food Chem. 2003 Dec 17;51(26):7568-75.
|
| Kinase Assay | Effect of coffee extracts on plasma fibrinolysis and platelet aggregation.[Pubmed: 21702337]Nihon Arukoru Yakubutsu Igakkai Zasshi. 2011 Apr;46(2):260-9.We have previously reported on study results showing that certain types of coffee have the activity to enhance fibrinolysis. |
| Structure Identification | J Agric Food Chem. 2002 Feb 27;50(5):1192-9.Alkylpyridiniums. 1. Formation in model systems via thermal degradation of trigonelline.[Pubmed: 11853503]Trigonelline is a well-known precursor of flavor/aroma compounds in coffee and undergoes significant degradation during roasting.
Sensors (Basel). 2011;11(4):4030-42.Surface plasmon resonance based biosensors for exploring the influence of alkaloids on aggregation of amyloid-β peptide.[Pubmed: 22163834]
|

Trigonelline hydrochloride Dilution Calculator

Trigonelline hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.7604 mL | 28.8018 mL | 57.6037 mL | 115.2074 mL | 144.0092 mL |
| 5 mM | 1.1521 mL | 5.7604 mL | 11.5207 mL | 23.0415 mL | 28.8018 mL |
| 10 mM | 0.576 mL | 2.8802 mL | 5.7604 mL | 11.5207 mL | 14.4009 mL |
| 50 mM | 0.1152 mL | 0.576 mL | 1.1521 mL | 2.3041 mL | 2.8802 mL |
| 100 mM | 0.0576 mL | 0.288 mL | 0.576 mL | 1.1521 mL | 1.4401 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Rifapentine
Catalog No.:BCC4937
CAS No.:61379-65-5
- Griffonilide
Catalog No.:BCN1271
CAS No.:61371-55-9
- erythro-Guaiacylglycerol-beta-O-4'-dehydrodisinapyl ether
Catalog No.:BCN7024
CAS No.:613684-55-2
- TNP-ATP triethylammonium salt
Catalog No.:BCC7373
CAS No.:61368-63-6
- VU 0364770
Catalog No.:BCC4597
CAS No.:61350-00-3
- Boc-D-Glu-OH
Catalog No.:BCC2606
CAS No.:61348-28-6
- Boc-D-Gln-OH
Catalog No.:BCC2607
CAS No.:61348-28-5
- Amoxicillin trihydrate
Catalog No.:BCC5168
CAS No.:61336-70-7
- Neoschaftoside
Catalog No.:BCN3053
CAS No.:61328-41-4
- Sulconazole Nitrate
Catalog No.:BCC4853
CAS No.:61318-91-0
- Boc-D-Arg(Tos)-OH
Catalog No.:BCC3070
CAS No.:61315-61-5
- Isoacteoside
Catalog No.:BCN4137
CAS No.:61303-13-7
- Naginata ketone
Catalog No.:BCN7801
CAS No.:6138-88-1
- 3-Amino-3-phenylpropionic acid
Catalog No.:BCC8609
CAS No.:614-19-7
- Procainamide HCl
Catalog No.:BCC5492
CAS No.:614-39-1
- 2-Hydroxycinnamic acid
Catalog No.:BCN5039
CAS No.:614-60-8
- 2,4-Dihydroxyphenylacetic acid
Catalog No.:BCN4139
CAS No.:614-82-4
- Rolipram
Catalog No.:BCC2282
CAS No.:61413-54-5
- Carmofur
Catalog No.:BCC1214
CAS No.:61422-45-5
- 2-Amino-3-Formylchromone
Catalog No.:BCC8526
CAS No.:61424-76-8
- Z-D-Ala-ol
Catalog No.:BCC2589
CAS No.:61425-27-2
- Bisabola-3,10-dien-2-one
Catalog No.:BCN7510
CAS No.:61432-71-1
- CIS-Resveratrol
Catalog No.:BCC8150
CAS No.:61434-67-1
- Lup-20(29)-ene-2alpha,3beta-diol
Catalog No.:BCN4612
CAS No.:61448-03-1
Alkylpyridiniums. 1. Formation in model systems via thermal degradation of trigonelline.[Pubmed:11853503]
J Agric Food Chem. 2002 Feb 27;50(5):1192-9.
Trigonelline is a well-known precursor of flavor/aroma compounds in coffee and undergoes significant degradation during roasting. This study investigates the major nonvolatile products that are procured after trigonelline has been subjected to mild pyrolysis conditions (220-250 degrees C) under atmospheric pressure. Various salt forms of trigonelline were also prepared and the thermally produced nonvolatiles analyzed by thin layer chromatography, liquid chromatography-electrospray ionization tandem mass spectrometry, and (1)H and (13)C nuclear magnetic resonance. Results revealed the decarboxylated derivative 1-methylpyridinium as a major product of certain salts, the formation of which is positively correlated to temperature from 220 to 245 degrees C. Moreover, Trigonelline hydrochloride afforded far greater amounts of 1-methylpyridinium compared to the monohydrate over the temperature range studied. Investigations into other potential quaternary amine products of trigonelline also indicate nucleophilic substitution reactions that lead to dialkylpyridiniums, albeit at concentration levels approximately 100-fold lower than those recorded for 1-methylpyridinium.
Effect of coffee extracts on plasma fibrinolysis and platelet aggregation.[Pubmed:21702337]
Nihon Arukoru Yakubutsu Igakkai Zasshi. 2011 Apr;46(2):260-9.
We have previously reported on study results showing that certain types of coffee have the activity to enhance fibrinolysis. This report covers the activity of 10 types of hot water extracts of coffee on human tissue-type plasminogen activator producing cells. Particularly strong activity (29-35 times the control amount) was observed for Blue Mountain, Yunnan and Kilimanjaro beans. It was found that the hot water extracts have anti-thrombin activity, and that coffee components have anti-platelet aggregation activity, although weak. It was revealed that there is no activity affecting tissue-type plasminogen activator producing cells in the coffee components chlorogenic acid, caffeine, quinic acid, Trigonelline hydrochloride, 5-(hydroxymethyl)-2-furfuryl and caffeic acid. It was also revealed that there is activity in fractions with a molecular weight of 10,000 or less. This could also be inferred from the fact that oral administration of such fractions of coffee to human subjects resulted in a shortening of their plasma ELT (p<0.05).
Surface plasmon resonance based biosensors for exploring the influence of alkaloids on aggregation of amyloid-beta peptide.[Pubmed:22163834]
Sensors (Basel). 2011;11(4):4030-42.
The main objective of the presented study was the development of a simple analytical tool for exploring the influence of naturally occurring compounds on the aggregation of amyloid-beta peptide (Abeta(40)) in order to find potential anti-neurodegenerative drugs. The gold discs used for surface plasmon resonance (SPR) measurements were modified with thioaliphatic acid. The surface functionalized with carboxylic groups was used for covalent attaching of Abeta(40) probe by creation of amide bonds in the presence of EDC/NHS. The modified SPR gold discs were used for exploring the Abeta(40) aggregation process in the presence of selected alkaloids: arecoline hydrobromide, pseudopelletierine hydrochloride, Trigonelline hydrochloride and alpha-lobeline hydrochloride. The obtained results were discussed with other parameters which govern the phenomenon studied such as lipophilicity/hydrophilicy and Abeta(40)-alkaloid association constants.
Effects of coffee components on the response of GABA(A) receptors expressed in Xenopus oocytes.[Pubmed:14664509]
J Agric Food Chem. 2003 Dec 17;51(26):7568-75.
The effects of both coffee components and coffee extract on the electrical responses of GABA(A) receptors expressed in Xenopus oocytes were studied by injecting cRNAs of the alpha(1) and beta(1) subunits of the bovine receptors. The aqueous extract of coffee dose-dependently inhibited the GABA-elicited responses, whereas the lipophilic extract of coffee by diethyl ether slightly potentiated it at low doses (0.1-0.4 microL/mL) but showed inhibition at high doses (0.5-0.8 microL/mL). Theophylline inhibited the response in a noncompetitive mechanism (K(i) = 0.55 mM), whereas theobromine and Trigonelline hydrochloride inhibited it in a competitive manner, K(i) = 3.8 and 13 mM, respectively. Benzothiazole, catechol, 2,4-dimethylstyrene, guaiacol, 1-octen-3-ol, sotolone, and 2,3,5-trimethylphenol potentiated the responses significantly. Potentiation elicited by guaiacol and sotolone was independent of GABA concentrations, whereas that by 1-octen-3-ol was dependent. When 1-octen-3-ol (100 mg/kg) was orally administered to mice prior to intraperitoneal administration of pentobarbital, the sleeping time of mice induced by pentobarbital increased significantly.


