Ibuprofen LysineCOX inhibitor CAS# 57469-77-9 |
- FK 3311
Catalog No.:BCC1576
CAS No.:116686-15-8
- Iguratimod
Catalog No.:BCC1641
CAS No.:123663-49-0
- Ibuprofen
Catalog No.:BCC3791
CAS No.:15687-27-1
- Celecoxib
Catalog No.:BCC1099
CAS No.:169590-42-5
- Etoricoxib
Catalog No.:BCC1565
CAS No.:202409-33-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 57469-77-9 | SDF | Download SDF |
| PubChem ID | 9841440 | Appearance | Powder |
| Formula | C19H32N2O4 | M.Wt | 352.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 71 mg/mL (201.43 mM) in Water | ||
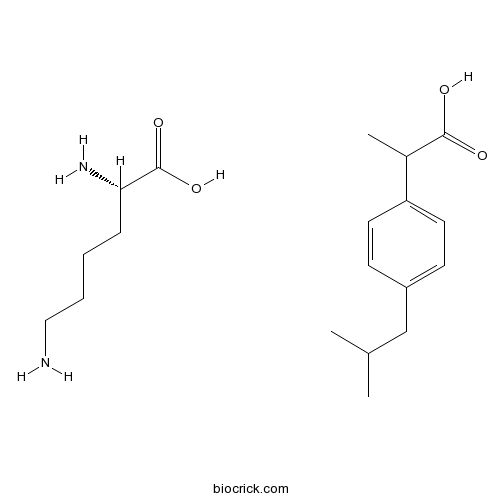
| Chemical Name | (2S)-2,6-diaminohexanoic acid;2-[4-(2-methylpropyl)phenyl]propanoic acid | ||
| SMILES | CC(C)CC1=CC=C(C=C1)C(C)C(=O)O.C(CCN)CC(C(=O)O)N | ||
| Standard InChIKey | IHHXIUAEPKVVII-ZSCHJXSPSA-N | ||
| Standard InChI | InChI=1S/C13H18O2.C6H14N2O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15;7-4-2-1-3-5(8)6(9)10/h4-7,9-10H,8H2,1-3H3,(H,14,15);5H,1-4,7-8H2,(H,9,10)/t;5-/m.0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ibuprofen Lysine Dilution Calculator

Ibuprofen Lysine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8371 mL | 14.1856 mL | 28.3712 mL | 56.7424 mL | 70.928 mL |
| 5 mM | 0.5674 mL | 2.8371 mL | 5.6742 mL | 11.3485 mL | 14.1856 mL |
| 10 mM | 0.2837 mL | 1.4186 mL | 2.8371 mL | 5.6742 mL | 7.0928 mL |
| 50 mM | 0.0567 mL | 0.2837 mL | 0.5674 mL | 1.1348 mL | 1.4186 mL |
| 100 mM | 0.0284 mL | 0.1419 mL | 0.2837 mL | 0.5674 mL | 0.7093 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ibuprofen Lysine (NeoProfen) is a non-selective COX inhibitor with an IC50 of 0.33 mM. It is known to have an antiplatelet effect, though Ibuprofen Lysine (NeoProfen) is relatively mild and short-lived when compared with that of aspirin or other better-kn
- H-Asn-OMe.HCl
Catalog No.:BCC2876
CAS No.:57461-34-4
- Stigmasta-3,5-diene
Catalog No.:BCC8254
CAS No.:4970-37-0
- 2-Epi-3a-epiburchellin
Catalog No.:BCN7013
CAS No.:57457-99-5
- p-Menth-8-ene-1,2-diol
Catalog No.:BCN5777
CAS No.:57457-97-3
- Resiniferatoxin
Catalog No.:BCC6951
CAS No.:57444-62-9
- Methylergometrine maleate
Catalog No.:BCC6691
CAS No.:57432-61-8
- (2S)-2alpha-(1,3-Benzodioxol-5-yl)-3,5-dihydro-5alpha-methoxy-3beta-methyl-5-allyl-2H-benzofuran-6-one
Catalog No.:BCN6606
CAS No.:57430-03-2
- 8-O-Acetylshanzhiside methyl ester
Catalog No.:BCN5776
CAS No.:57420-46-9
- Fexaramine
Catalog No.:BCC7412
CAS No.:574013-66-4
- Fraxetin
Catalog No.:BCN5903
CAS No.:574-84-5
- Isoflavone
Catalog No.:BCN8508
CAS No.:574-12-9
- Benzoin ethyl ether
Catalog No.:BCC8855
CAS No.:574-09-4
- 16-Deoxysaikogenin F
Catalog No.:BCN6414
CAS No.:57475-62-4
- BRL-54443
Catalog No.:BCC5047
CAS No.:57477-39-1
- Carbasalate calcium
Catalog No.:BCC8904
CAS No.:5749-67-7
- Angeflorin
Catalog No.:BCN6656
CAS No.:57498-69-8
- Carpachromene
Catalog No.:BCN5779
CAS No.:57498-96-1
- 3,4'-Di-O-methylellagic acid
Catalog No.:BCN3710
CAS No.:57499-59-9
- 3-Benzalphthalide
Catalog No.:BCC8621
CAS No.:575-61-1
- 6-Benzyloxypurine
Catalog No.:BCC8770
CAS No.:57500-07-9
- 2-Methylsulfanylpyrimidin-4(3H)-one
Catalog No.:BCC8582
CAS No.:5751-20-2
- H-His(Nτ-Me)-OMe.2HCl
Catalog No.:BCC2958
CAS No.:57519-09-2
- Notoginsenoside S
Catalog No.:BCN8371
CAS No.:575446-95-6
- SNOG
Catalog No.:BCC6714
CAS No.:57564-91-7
Reversibly changing a painkiller structure: a hot topic for a cold case--ibuprofen lysine salt.[Pubmed:25659531]
J Pharm Biomed Anal. 2015 Mar 25;107:394-402.
Ibuprofen Lysine salt can undergo a fully reversible, thermally induced phase transition into a different enantiotropically related polymorphic form. The structures of both the high and low temperature phases were solved using state-of-the-art X-ray powder diffraction methods, showing many similarities both in the molecular conformation and in the crystal packing. The full structural analysis and comparison of the two crystal structures allowed to understand the mechanism of the phase transition and explain its reversible nature in what appears to be a rare case of isosymmetric temperature-driven phase transformation of an organic solid.
Severe pulmonary hypertension with therapeutic L-lysine ibuprofen in 2 preterm neonates.[Pubmed:22492771]
Pediatrics. 2012 May;129(5):e1360-3.
Persistently patent ductus arteriosus (PDA), affecting approximately one-third of all very low birth weight infants, can lead to significant morbidity and mortality. Recently, ibuprofen has been recommended over indomethacin to close PDAs because of a reduction in risk of necrotizing enterocolitis. Pulmonary hypertension is a rare but potentially fatal complication of ibuprofen administration in preterm infants. We report 2 infants who developed this complication after receiving therapeutic L-lysine ibuprofen preparation for the PDA closure. The first infant, 1 of twins weighing 640 g, was born at 24 weeks' gestation. The second infant, born at 26 weeks' gestation, was small for gestational age, weighing 439 g. In both cases, ibuprofen was initiated after echocardiographic confirmation of a moderate-sized to large PDA and an otherwise normal intracardiac anatomy. Both infants had echocardiographic evidence of increased pulmonary vascular resistance but shunting across the PDA was left to right. The infants deteriorated within 48 to 72 hours, and repeat echocardiograms revealed evidence of severe pulmonary hypertension. Both infants died of refractory hypotension and hypoxemia. When considering the use of ibuprofen therapy for PDA closure, clinicians should keep in mind the potential serious complication of pulmonary hypertension, even if a shunt across the PDA is left to right.
Characterization of alginate beads loaded with ibuprofen lysine salt and optimization of the preparation method.[Pubmed:24177314]
Int J Pharm. 2014 Jan 2;460(1-2):181-8.
The parameters influencing alginate ionotropic gelation and the production of alginate beads loaded with hydrosoluble Ibuprofen Lysine salt (IBU-L) were studied, as well as the optimization of the method for its attainment. A three-factor and three-level factorial design (3(3)) was carried out to determine the influence of three experimental variables: polymer concentration, CaCl2 concentration, and curing time on the dependent variables drug load and encapsulation efficiency. The effect of the pH used in the preparation bath was also evaluated. Concentrations of CaCl2 and pH of gelling bath were seen to affect bead formation and stability as well as their ability to properly entrap the drug. In this work, IBU-L was used as a model of a non-steroidal anti-inflammatory drug with good solubility in alginate solutions. IBU-L was successfully encapsulated in alginate beads obtained by the ionotropic gelation method. The obtained alginate matrixes are able to modify the release of the entrapped IBU-L and this occurs in a pH-sensitive way that can be correlated with the swelling behaviour of the alginate-produced beads. Morphological characteristics were evaluated by means of scanning electron microscopy.
Enantioselective adsorption of ibuprofen and lysine in metal-organic frameworks.[Pubmed:25090236]
Chem Commun (Camb). 2014 Sep 25;50(74):10849-52.
This study reveals the efficient enantiomeric separation of bioactive molecules in the liquid phase. Chiral structure HMOF-1 separates racemic mixtures whereas heteroselectivity is observed for scalemic mixtures of ibuprofen using non-chiral MIL-47 and MIL-53. Lysine enantiomers are only separated by HMOF-1. These separations are controlled by the tight confinement of the molecules.


