Hoechst 33258 analog 5Blue fluorescent dyes CAS# 23491-55-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23491-55-6 | SDF | Download SDF |
| PubChem ID | 10321921 | Appearance | Powder |
| Formula | C29H26N6 | M.Wt | 458.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | 25℃: DMSO or water Protect from light | ||
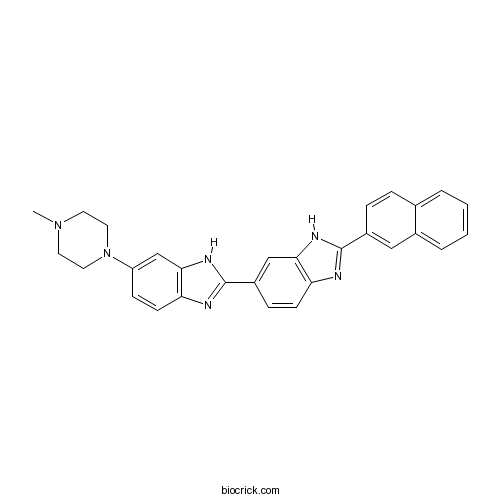
| Chemical Name | 6-(4-methylpiperazin-1-yl)-2-(2-naphthalen-2-yl-3H-benzimidazol-5-yl)-1H-benzimidazole | ||
| SMILES | CN1CCN(CC1)C2=CC3=C(C=C2)N=C(N3)C4=CC5=C(C=C4)N=C(N5)C6=CC7=CC=CC=C7C=C6 | ||
| Standard InChIKey | FJXFKDPHPUCTJZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H26N6/c1-34-12-14-35(15-13-34)23-9-11-25-27(18-23)33-29(31-25)22-8-10-24-26(17-22)32-28(30-24)21-7-6-19-4-2-3-5-20(19)16-21/h2-11,16-18H,12-15H2,1H3,(H,30,32)(H,31,33) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Hoechst 33258 analog 5 Dilution Calculator

Hoechst 33258 analog 5 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1807 mL | 10.9037 mL | 21.8074 mL | 43.6148 mL | 54.5185 mL |
| 5 mM | 0.4361 mL | 2.1807 mL | 4.3615 mL | 8.723 mL | 10.9037 mL |
| 10 mM | 0.2181 mL | 1.0904 mL | 2.1807 mL | 4.3615 mL | 5.4518 mL |
| 50 mM | 0.0436 mL | 0.2181 mL | 0.4361 mL | 0.8723 mL | 1.0904 mL |
| 100 mM | 0.0218 mL | 0.109 mL | 0.2181 mL | 0.4361 mL | 0.5452 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: N/A Hoechst stains are part of a family of blue fluorescent dyes used to stain DNA. These Bis-benzimides were originally developed by Hoechst AG, which numbered all their compounds so that the dye Hoechst 33342 is the 33342nd compound made by the company. There are three related Hoechst stains: Hoechst 33258, Hoechst 33342, and Hoechst 34580. The dyes Hoechst 33258 and Hoechst 33342 are the ones most commonly used and they have similarexcitation/emission spectra. Both dyes are excited by ultraviolet light at around 350 nm, and both emit blue/cyan fluorescent light around anemission maximum at 461 nm. Unbound dye has its maximum fluorescence emission in the 510-540 nm range. Hoechst dyes are soluble in water and in organic solvents such as dimethyl formamide or dimethyl sulfoxide. Concentrations can be achieved of up to 10 mg/mL. Aqueous solutions are stable at 2-6 °C for at least six months when protected from light. For long-term storage the solutions are instead frozen at ≤-20 °C. The dyes bind to the minor groove of double-stranded DNA with a preference for sequences rich in adenine andthymine. Although the dyes can bind to all nucleic acids, AT-rich double-stranded DNA strands enhance fluorescence considerably. Hoechst dyes are cell-permeable and can bind to DNA in live or fixed cells. Therefore, these stains are often called supravital, which means that cells survive a treatment with these compounds. Cells that express specific ATP-binding cassette transporter proteins can also actively transport these stains out of their cytoplasm. in vitro: N/A in vivo: N/A Clinical trial: N/A
- Hoechst 33258 analog 2
Catalog No.:BCC1625
CAS No.:23491-54-5
- Hoechst 33342
Catalog No.:BCC1629
CAS No.:23491-52-3
- Hoechst 33258
Catalog No.:BCC1623
CAS No.:23491-45-4
- 2-Amino-5-mercapto-1,3,4-thiadiazole
Catalog No.:BCC8536
CAS No.:2349-67-9
- U 99194 maleate
Catalog No.:BCC7029
CAS No.:234757-41-6
- 2-Palmitoylglycerol
Catalog No.:BCC7289
CAS No.:23470-00-0
- trans-Khellactone
Catalog No.:BCN6920
CAS No.:23458-04-0
- Decursinol
Catalog No.:BCN2638
CAS No.:23458-02-8
- alpha-Spinasterone
Catalog No.:BCN5086
CAS No.:23455-44-9
- Trenbolone cyclohexylmethylcarbonate
Catalog No.:BCC9185
CAS No.:23454-33-3
- Alternariol monomethyl ether
Catalog No.:BCN7384
CAS No.:23452-05-3
- Physcion 1-glucoside
Catalog No.:BCN8170
CAS No.:23451-01-6
- Peimine
Catalog No.:BCN1094
CAS No.:23496-41-5
- Axillaridine
Catalog No.:BCN2060
CAS No.:23506-96-9
- MEN 11270
Catalog No.:BCC6094
CAS No.:235082-52-7
- Humulon
Catalog No.:BCC8186
CAS No.:23510-81-8
- 8-Gingerol
Catalog No.:BCN5921
CAS No.:23513-08-8
- 6-Gingerol
Catalog No.:BCN1030
CAS No.:23513-14-6
- 10-Gingerol
Catalog No.:BCN5922
CAS No.:23513-15-7
- (-)-licarin A
Catalog No.:BCN5087
CAS No.:23518-30-1
- Vomifoliol
Catalog No.:BCN5088
CAS No.:23526-45-6
- 5-Aza-2'-deoxycytidine
Catalog No.:BCN2169
CAS No.:2353-33-5
- 8-Debenzoylpaeoniflorin
Catalog No.:BCC8787
CAS No.:23532-11-8
- Daunorubicin HCl
Catalog No.:BCC5083
CAS No.:23541-50-6
Identification of the key pathway of oxazolinoanthracyclines mechanism of action in cells derived from human solid tumors.[Pubmed:27780733]
Toxicol Appl Pharmacol. 2016 Dec 15;313:159-169.
Oxazolinodoxorubicin (O-DOX) and oxazolinodaunorubicin (O-DAU) are novel anthracycline derivatives with a modified daunosamine moiety. In the present study, we evaluated the cytotoxicities, genotoxicities and abilities of O-DOX and O-DAU to induce apoptosis in cancer cell lines (SKOV-3; A549; HepG2), and compared the results with their parent drugs. We assessed antiproliferative activity by MTT assay. We evaluated apoptosis-inducing ability by double-staining with fluorescent probes (Hoechst 33258/propidium iodide), and by determining expression levels of genes involved in programmed cell death by reverse transcription-polymerase chain reaction. Genotoxicities of the compounds were tested by comet assays. Oxazolinoanthracyclines demonstrated high anti-tumor activity. O-DOX had significantly higher cytotoxicity, apoptosis-inducing ability, and genotoxicity compared with parental doxorubicin (DOX) in all tested conditions, while O-DAU activity differed among cell lines. The mechanism of oxazoline analog action appeared to involve the mitochondrial pathway of programmed cell death. These results provide further information about oxazoline derivatives of commonly used anthracycline chemotherapy agents. O-DOX and O-DAU have the ability to induce apoptosis in tumor cells.
Dynamic proliferation assessment in flow cytometry.[Pubmed:20853345]
Curr Protoc Cell Biol. 2010 Sep;Chapter 8:Unit 8.6.1-23.
Dynamic proliferation assessment via flow cytometry is legitimately supposed to be the most powerful tool for recording cell cycle kinetics in-vitro. The preeminent feature is a single cell-based multi-informative analysis by temporal high-resolution. Flow cytometric approaches are based on labeling of proliferating cells via thymidine substitution by a base analog (e.g., 5-bromo-2'-deoxyuridine, BrdU) that is added to cell cultures either for a short period of time (pulse labeling) or continuously until cell harvesting. This unit describes the alternative use of the thymidine analog 5-ethynyl-2'-deoxyuridine (EdU) in place of BrdU for three different applications: (1) dynamic proliferation assessment by EdU pulse cell labeling; (2) the same approach as (1) but in combination with live/dead cell discrimination; and (3) dynamic cell cycle analysis based on continuous cell labeling with EdU and Hoechst fluorochrome quenching. In contrast to the detection of BrdU incorporation, EdU-positive cells can be identified by taking advantage of click chemistry, which facilitates a simplified and fast cell preparation. Further analysis options but also limitations of the utilization of EdU are discussed.
Synthesis and antitumor activities of novel rhein alpha-aminophosphonates conjugates.[Pubmed:24378217]
Bioorg Med Chem Lett. 2014 Jan 15;24(2):501-7.
Several rhein alpha-aminophosphonates conjugates (5a-5q) were synthesized and evaluated for in vitro cytotoxicity against HepG-2, CNE, Spca-2, Hela and Hct-116 cell lines. Some compounds showed relatively high cytotoxicity. Especially, compound 5i exhibited the strongest cytotoxicity against Hct-116 cells (IC50 was 5.32 muM). All the synthesized compounds exhibited low cytotoxicity against HUVEC cells. The mechanism of compound 5i was preliminarily investigated by Hoechst 33258 staining, JC-1 mitochondrial membrane potential staining and flow cytometry, which indicated that the compound 5i induced apoptosis in Hct-116 cancer cells. Cell cycle analysis showed that these compound 5i mainly arrested Hct-116 cells in G1 stage. The effects of 5i on the activation of caspases expression indicated that 5i might induce apoptosis via the membrane death receptor pathways. In addition, the binding properties of a model analog 5i to DNA were investigated by methods (UV-vis, fluorescence, CD spectroscopy and FRET-melting) in compare with that of rhein. Results indicated that 5i showed moderate ability to interact ct-DNA.
Da0324, an inhibitor of nuclear factor-kappaB activation, demonstrates selective antitumor activity on human gastric cancer cells.[Pubmed:27042000]
Drug Des Devel Ther. 2016 Mar 2;10:979-95.
BACKGROUND: The transcription factor nuclear factor-kappaB (NF-kappaB) is constitutively activated in a variety of human cancers, including gastric cancer. NF-kappaB inhibitors that selectively kill cancer cells are urgently needed for cancer treatment. Curcumin is a potent inhibitor of NF-kappaB activation. Unfortunately, the therapeutic potential of curcumin is limited by its relatively low potency and poor cellular bioavailability. In this study, we presented a novel NF-kappaB inhibitor named Da0324, a synthetic asymmetric mono-carbonyl analog of curcumin. The purpose of this study is to research the expression of NF-kappaB in gastric cancer and the antitumor activity and mechanism of Da0324 on human gastric cancer cells. METHODS: The expressions between gastric cancer tissues/cells and normal gastric tissues/cells of NF-kappaB were evaluated by Western blot. The inhibition viability of compounds on human gastric cancer cell lines SGC-7901, BGC-823, MGC-803, and normal gastric mucosa epithelial cell line GES-1 was assessed with the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay. Absorption spectrum method and high-performance liquid chromatography method detected the stability of the compound in vitro. The compound-induced changes of inducible NF-kappaB activation in the SGC-7901 and BGC-823 cells were examined by Western blot analysis and immunofluorescence methods. The antitumor activity of compound was performed by clonogenic assay, matrigel invasion assay, flow cytometric analysis, Western blot analysis, and Hoechst 33258 staining assay. RESULTS: High levels of p65 were found in gastric cancer tissues and cells. Da0324 displayed higher growth inhibition against several types of gastric cancer cell lines and showed relatively low toxicity to GES-1. Moreover, Da0324 was more stable than curcumin in vitro. Western blot analysis and immunofluorescence methods showed that Da0324 blocked NF-kappaB activation. In addition, Da0324 significantly inhibited tumor proliferation and invasion, arrested the cell cycle, and induced apoptosis in vitro. CONCLUSION: The asymmetric mono-carbonyl analog of curcumin Da0324 exhibited significantly improved antigastric cancer activity. Da0324 may be a promising NF-kappaB inhibitor for the selective targeting of cancer cells. However, further studies are needed in animals to validate these findings for the therapeutic use of Da0324.
Norcantharidin induced DU145 cell apoptosis through ROS-mediated mitochondrial dysfunction and energy depletion.[Pubmed:24367681]
PLoS One. 2013 Dec 19;8(12):e84610.
Norcantharidin (NCTD), a demethylated analog of cantharidin derived from blister beetles, has attracted considerable attentions in recent years due to their definitely toxic properties and the noteworthy advantages in stimulating bone marrow and increasing the peripheral leukocytes. Hence, it is worth studying the anti-tumor effect of NCTD on human prostate cancer cells DU145. It was found that after the treatment of NCTD with different concentrations (25-100 muM), the cell proliferation was significantly inhibited, which led to the appearance of micronucleus (MN). Moreover, the cells could be killed in a dose-/time-dependent manner along with the reduction of PCNA (proliferating cell nuclear antigen) expression, destruction of mitochondrial membrane potential (MMP), down-regulation of MnSOD, induction of ROS, depletion of ATP, and activation of AMPK (Adenosine 5'-monophosphate -activated protein kinase) . In addition, a remarkable release of cytochrome c was found in the cells exposed to 100 muM NCTD and exogenous SOD-PEG could eliminate the generation of NCTD-induced MN. In conclusion, our studies indicated that NCTD could induce the collapse of MMP and mitochondria dysfunction. Accumulation of intercellular ROS could eventually switch on the apoptotic pathway by causing DNA damage and depleting ATP.


