2-PalmitoylglycerolCAS# 23470-00-0 |
- ABT-751 (E7010)
Catalog No.:BCC1085
CAS No.:141430-65-1
- CHM 1
Catalog No.:BCC2387
CAS No.:154554-41-3
- Podophyllotoxin
Catalog No.:BCN5957
CAS No.:518-28-5
- D-64131
Catalog No.:BCC1510
CAS No.:74588-78-6
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23470-00-0 | SDF | Download SDF |
| PubChem ID | 123409 | Appearance | Powder |
| Formula | C19H38O4 | M.Wt | 330.51 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 2-Palm-Gl, 2-PG | ||
| Solubility | Soluble to 100 mM in ethanol and to 100 mM in DMSO | ||
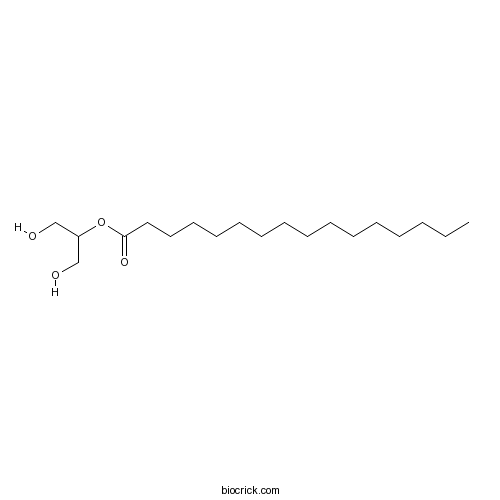
| Chemical Name | 1,3-dihydroxypropan-2-yl hexadecanoate | ||
| SMILES | CCCCCCCCCCCCCCCC(=O)OC(CO)CO | ||
| Standard InChIKey | BBNYCLAREVXOSG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H38O4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-19(22)23-18(16-20)17-21/h18,20-21H,2-17H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous fatty acid glycerol ester that enhances activity of 2-arachidonylglycerol. Does not bind to CB1 or CB2 cannabinoid receptors, but potentiates the apparent binding of 2-AG and increases its ability to inhibit adenylyl cyclase. Enhances in vivo cannabinoid effects of 2-AG following combined administration with 2-linoleoylglycerol. |

2-Palmitoylglycerol Dilution Calculator

2-Palmitoylglycerol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0256 mL | 15.1281 mL | 30.2563 mL | 60.5125 mL | 75.6407 mL |
| 5 mM | 0.6051 mL | 3.0256 mL | 6.0513 mL | 12.1025 mL | 15.1281 mL |
| 10 mM | 0.3026 mL | 1.5128 mL | 3.0256 mL | 6.0513 mL | 7.5641 mL |
| 50 mM | 0.0605 mL | 0.3026 mL | 0.6051 mL | 1.2103 mL | 1.5128 mL |
| 100 mM | 0.0303 mL | 0.1513 mL | 0.3026 mL | 0.6051 mL | 0.7564 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- trans-Khellactone
Catalog No.:BCN6920
CAS No.:23458-04-0
- Decursinol
Catalog No.:BCN2638
CAS No.:23458-02-8
- alpha-Spinasterone
Catalog No.:BCN5086
CAS No.:23455-44-9
- Trenbolone cyclohexylmethylcarbonate
Catalog No.:BCC9185
CAS No.:23454-33-3
- Alternariol monomethyl ether
Catalog No.:BCN7384
CAS No.:23452-05-3
- Physcion 1-glucoside
Catalog No.:BCN8170
CAS No.:23451-01-6
- Irisolidone
Catalog No.:BCN8496
CAS No.:2345-17-7
- Swertianolin
Catalog No.:BCN2759
CAS No.:23445-00-3
- Tetrahydropiperin
Catalog No.:BCN6708
CAS No.:23434-88-0
- 7-Isopentenyloxy-gamma-fagarine
Catalog No.:BCN5085
CAS No.:23417-92-7
- Phalaenopsine La
Catalog No.:BCN2015
CAS No.:23412-99-9
- Phalaenopsine T
Catalog No.:BCN2014
CAS No.:23412-97-7
- U 99194 maleate
Catalog No.:BCC7029
CAS No.:234757-41-6
- 2-Amino-5-mercapto-1,3,4-thiadiazole
Catalog No.:BCC8536
CAS No.:2349-67-9
- Hoechst 33258
Catalog No.:BCC1623
CAS No.:23491-45-4
- Hoechst 33342
Catalog No.:BCC1629
CAS No.:23491-52-3
- Hoechst 33258 analog 2
Catalog No.:BCC1625
CAS No.:23491-54-5
- Hoechst 33258 analog 5
Catalog No.:BCC1627
CAS No.:23491-55-6
- Peimine
Catalog No.:BCN1094
CAS No.:23496-41-5
- Axillaridine
Catalog No.:BCN2060
CAS No.:23506-96-9
- MEN 11270
Catalog No.:BCC6094
CAS No.:235082-52-7
- Humulon
Catalog No.:BCC8186
CAS No.:23510-81-8
- 8-Gingerol
Catalog No.:BCN5921
CAS No.:23513-08-8
- 6-Gingerol
Catalog No.:BCN1030
CAS No.:23513-14-6
Facile preparation of magnetic carbon nanotubes-immobilized lipase for highly efficient synthesis of 1,3-dioleoyl-2-palmitoylglycerol-rich human milk fat substitutes.[Pubmed:28317752]
Food Chem. 2017 Aug 1;228:476-483.
In this study, Candida lipolytica lipase (CLL) was immobilized on magnetic multi-walled carbon nanotubes (mMWCNTs) via hydrophobic and cation-exchange interaction. The resultant immobilized CLL showed much better thermal stability, biocatalyst activity and easier recycling than the free form. A method for efficient enzymatic acidolysis of tripalmitin (PPP) with oleic acid (OA), to produce OPO-rich TAGs, was developed, using the immobilized CLL as the biocatalyst. Under optimized conditions (2% water, 20mg/ml enzyme, 1:6 PPP/OA, 50 degrees C, 2h), the content of OPO in the final product reached 46.5%. CLL@mMWCNTs had a better activity and manipulative stability than commercial lipases. More importantly, the feasibility of CLL@mMWCNTs was also validated in the practical production of OPO-rich TAGs, using lard and restructured palm oil as the raw material. These results suggest that CLL@mMWCNTs is a promising biocatalyst for the OPO-rich TAGs production and will be helpful for the infant formula industry.
Immobilization, Regiospecificity Characterization and Application of Aspergillus oryzae Lipase in the Enzymatic Synthesis of the Structured Lipid 1,3-Dioleoyl-2-Palmitoylglycerol.[Pubmed:26218640]
PLoS One. 2015 Jul 28;10(7):e0133857.
The enzymatic synthesis of 1,3-dioleoyl-2-Palmitoylglycerol (OPO), one of the main components of human milk fats, has been hindered by the relatively high cost of sn-1,3-specific lipases and the deficiency in biocatalyst stability. The sn-1,3-specific lipase from Aspergillus oryzae (AOL) is highly and efficiently immobilized with the polystyrene-based hydrophobic resin D3520, with a significant 49.54-fold increase in specific lipase activity compared with the AOL powder in catalyzing the synthesis of OPO through the acidolysis between palm stearin and oleic acid (OA). The optimal immobilization conditions were investigated, including time course, initial protein concentration and solution pH. The sn-1,3 specificity of lipases under different immobilization conditions was evaluated and identified as positively associated with the lipase activity, and the pH of the immobilization solution influenced the regiospecificity and synthetic activity of these lipases. Immobilized AOL D3520, as the biocatalyst, was used for the enzymatic synthesis of the structured lipid OPO through the acidolysis between palm stearin and OA. The following conditions were optimized for the synthesis of structured lipid OPO: 65 degrees C temperature; 1:8 substrate molar ratio between palm stearin and OA; 8% (w/w) enzyme load; 3.5% water content of the immobilized lipase; and 1 h reaction time. Under these conditions, highly efficient C52 production (45.65%) was achieved, with a tripalmitin content of 2.75% and a sn-2 palmitic acid (PA) proportion of 55.08% in the system.
Ultrasonic pretreatment in lipase-catalyzed synthesis of structured lipids with high 1,3-dioleoyl-2-palmitoylglycerol content.[Pubmed:25453210]
Ultrason Sonochem. 2015 Mar;23:100-8.
Production of structured lipid 1,3-dioleoyl-2-Palmitoylglycerol (OPO), from tripalmitin (PPP) and oleic acid (OA) using lipases and ultrasonic pretreatment was conducted. Factors influencing both the ultrasonic conditions and enzymatic reaction were investigated. Optimum conditions could be attained with 6 min pretreatment time, 50% ultrasonic power, 3 s/9 s (work/pause) cycle of ultrasonic pulse, 1:8 PPP/OA molar ratio, 12% enzyme dosage and 50 degrees C temperature of. At the optimum conditions, the OPO yield of 51.8% could be achieved in 4h. Studies showed that the OPO content increased to 35.9% in 1h with ultrasonic pretreatment, in comparison to 4h without ultrasonic pretreatment. Reuse of Lipozyme RM IM for 10 cycles under ultrasonic irradiation did not cause essential damage to its lipase activity. Reaction kinetic model fitted well with the proposed Ping-Pong mechanism. The apparent kinetic constant (Vm'/K(2)) of ultrasound pretreatment reaction was 2.52 times higher than the conventional mechanical stirring, indicating that ultrasound pretreatment enhanced the substrates affinity to the enzyme. This study confirmed that ultrasonic pretreatment was more efficient in OPO production than conventional mechanical agitation.
Where's my entourage? The curious case of 2-oleoylglycerol, 2-linolenoylglycerol, and 2-palmitoylglycerol.[Pubmed:27117667]
Pharmacol Res. 2016 Aug;110:173-180.
2-Arachidonoylglycerol (2-AG) is the most abundant endogenous cannabinoid in the brain and an agonist at two cannabinoid receptors (CB1 and CB2). The synthesis, degradation and signaling of 2-AG have been investigated in detail but its relationship to other endogenous monoacylglycerols has not been fully explored. Three congeners that have been isolated from the CNS are 2-linoleoylglycerol (2-LG), 2-oleoylglycerol (2-OG), and 2-Palmitoylglycerol (2-PG). These lipids do not orthosterically bind to cannabinoid receptors but are reported to potentiate the activity of 2-AG, possibly through inhibition of 2-AG degradation. This phenomenon has been dubbed the 'entourage effect' and has been proposed to regulate synaptic activity of 2-AG. To clarify the activity of these congeners of 2-AG we tested them in neuronal and cell-based signaling assays. The signaling profile for these compounds is inconsistent with an entourage effect. None of the compounds inhibited neurotransmission via CB1 in autaptic neurons. Interestingly, each failed to potentiate 2-AG-mediated depolarization-induced suppression of excitation (DSE), behaving instead as antagonists. Examining other signaling pathways we found that 2-OG interferes with agonist-induced CB1 internalization while 2-PG modestly internalizes CB1 receptors. However in tests of pERK, cAMP and arrestin recruitment, none of the acylglycerols altered CB1 signaling. Our results suggest 1) that these compounds do not serve as entourage compounds under the conditions examined, and 2) that they may instead serve as functional antagonists. Our results suggest that the relationship between 2-AG and its congeners is more nuanced than previously appreciated.
Endocannabinoids and related fatty acid derivatives in pain modulation.[Pubmed:12505698]
Chem Phys Lipids. 2002 Dec 31;121(1-2):159-72.
The brain produces at least five compounds that possess sub-micromolar affinity for cannabinoid receptors: anandamide, 2-arachidonoylglycerol, noladin ether, virodhamine, and N-arachidonoyldopamine (NADA). One function of these and/or related compounds is to suppress pain sensitivity. Much evidence supports a role of endocannabinoids in pain modulation in general, and some evidence points to the role of particular endocannabinoids. Related endogenous fatty acid derivatives such as oleamide, palmitoylethanolamide, 2-lineoylglycerol, 2-Palmitoylglycerol, and a family of arachidonoyl amino acids may interact with endocannabinoids in the modulation of pain sensitivity.
2-Arachidonylglycerol, an endogenous cannabinoid, inhibits tumor necrosis factor-alpha production in murine macrophages, and in mice.[Pubmed:11011050]
Eur J Pharmacol. 2000 Oct 6;406(1):R5-7.
2-Arachidonylglycerol (2-AG) inhibits the production in vitro of tumor necrosis factor-alpha (TNF-alpha) by mouse macrophages, as well as in mice. It has no effect on the production of nitric oxide (NO). The effect on TNF-alpha is enhanced when 2-AG is administered together with 2-linoleylglycerol (2-Lino-G) and 2-palmitylglycerol (2-PalmG), an 'entourage effect' previously noted in several behavioral and binding assays. 2-AG also suppresses the formation of radical oxygen intermediates.
An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity.[Pubmed:9721036]
Eur J Pharmacol. 1998 Jul 17;353(1):23-31.
2-Arachidonoyl-glycerol (2-Ara-GI) has been isolated from various tissues and identified as an endogenous ligand for both cannabinoid receptors, CB1 and CB2. Here we report that in spleen, as in brain and gut, 2-Ara-GI is accompanied by several 2-acyl-glycerol esters, two major ones being 2-linoleoyl-glycerol (2-Lino-Gl) and 2-palmitoyl-glycerol (2-Palm-Gl). These two esters do not bind to the cannabinoid receptors, nor do they inhibit adenylyl cyclase via either CB1 or CB2; however, they significantly potentiate the apparent binding of 2-Ara-Gl and its apparent capacity to inhibit adenylyl cyclase. Together these esters also significantly potentiate 2-Ara-Gl inhibition of motor behavior, immobility on a ring, analgesia on a hot plate and hypothermia caused by 2-Ara-Gl in mice. 2-Lino-Gl, but not 2-Palm-GI, significantly inhibits the inactivation of 2-Ara-Gl by neuronal and basophilic cells. These data indicate that the biological activity of 2-Ara-Gl can be increased by related, endogenous 2-acyl-glycerols, which alone show no significant activity in any of the tests employed. This effect ('entourage effect') may represent a novel route for molecular regulation of endogenous cannabinoid activity.


