FerulamideCAS# 61012-31-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 61012-31-5 | SDF | Download SDF |
| PubChem ID | 6433734 | Appearance | Powder |
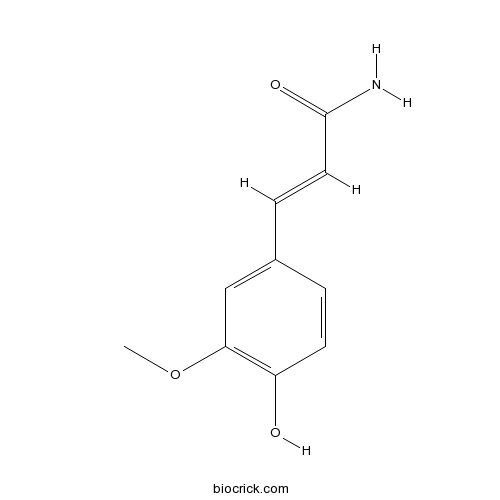
| Formula | C10H11NO3 | M.Wt | 193.2 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enamide | ||
| SMILES | COC1=C(C=CC(=C1)C=CC(=O)N)O | ||
| Standard InChIKey | YYAJJKZSQWOLIP-HWKANZROSA-N | ||
| Standard InChI | InChI=1S/C10H11NO3/c1-14-9-6-7(2-4-8(9)12)3-5-10(11)13/h2-6,12H,1H3,(H2,11,13)/b5-3+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Ferulamide derivatives show potent inhibitory activity against arachidonic acid-induced platelet aggregation. |
| Targets | PAFR |

Ferulamide Dilution Calculator

Ferulamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.176 mL | 25.8799 mL | 51.7598 mL | 103.5197 mL | 129.3996 mL |
| 5 mM | 1.0352 mL | 5.176 mL | 10.352 mL | 20.7039 mL | 25.8799 mL |
| 10 mM | 0.5176 mL | 2.588 mL | 5.176 mL | 10.352 mL | 12.94 mL |
| 50 mM | 0.1035 mL | 0.5176 mL | 1.0352 mL | 2.0704 mL | 2.588 mL |
| 100 mM | 0.0518 mL | 0.2588 mL | 0.5176 mL | 1.0352 mL | 1.294 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Succinylcholine Chloride Dihydrate
Catalog No.:BCC4564
CAS No.:6101-15-1
- Isoacetovanillone
Catalog No.:BCN7166
CAS No.:6100-74-9
- Tenuazonic acid
Catalog No.:BCN1859
CAS No.:610-88-8
- H-Leu-OH
Catalog No.:BCC2968
CAS No.:61-90-5
- Zoxazolamine
Catalog No.:BCC4751
CAS No.:61-80-3
- 4-Aminohippuric Acid
Catalog No.:BCC4753
CAS No.:61-78-9
- Phenylephrine HCl
Catalog No.:BCC4335
CAS No.:61-76-7
- Mefenamic Acid
Catalog No.:BCC4433
CAS No.:61-68-7
- Papaverine Hydrochloride
Catalog No.:BCC8348
CAS No.:61-25-6
- Adenosine 5'-monophosphate
Catalog No.:BCC8809
CAS No.:61-19-8
- Dibucaine (Cinchocaine) HCl
Catalog No.:BCC3760
CAS No.:61-12-1
- 2-Methoxycinnamic acid
Catalog No.:BCN5038
CAS No.:6099-03-2
- c-JUN peptide
Catalog No.:BCC8085
CAS No.:610273-01-3
- Teicoplanin
Catalog No.:BCC4731
CAS No.:61036-62-2
- N-Desmethylclozapine
Catalog No.:BCC6887
CAS No.:6104-71-8
- L-Thyroxine sodium salt pentahydrate
Catalog No.:BCC4283
CAS No.:6106-07-6
- Boc-His(Nτ-Me)-OH
Catalog No.:BCC2684
CAS No.:61070-22-2
- Isobonducellin
Catalog No.:BCN4130
CAS No.:610778-85-3
- Icotinib
Catalog No.:BCC4473
CAS No.:610798-31-7
- 4-Phenyl-1,2,3,4-tetrahydroisoquinoline hydrochloride
Catalog No.:BCC6769
CAS No.:6109-35-9
- Tectoridin
Catalog No.:BCN1020
CAS No.:611-40-5
- (R)-Mandelic acid
Catalog No.:BCN8532
CAS No.:611-71-2
- Bromhexine hydrochloride
Catalog No.:BCC8898
CAS No.:611-75-6
- Epipterosin L 2'-O-glucoside
Catalog No.:BCN4614
CAS No.:61117-89-3
A new alkaloid from Portulaca oleracea L. and its antiacetylcholinesterase activity.[Pubmed:29665731]
Nat Prod Res. 2018 Apr 18:1-8.
A new alkaloid, (10E, 12E)-9-ureidooctadeca-10, 12-dienoic acid, named oleraurea (1) and 10 known compounds, p-hydroxybenzaldehyde (2), p-hydroxybenzoic acid (3), p-hydroxyacetophenone (4), benzamide (5), (E)-p-coumaramide (6), (E)-Ferulamide (7), soyalkaloid A (8), beta-carboline-3-carboxylic acid (9), 2, 3, 4, 9-tetrahydro-1H-pyrido [3, 4-b] indole-3-carboxylic acid (10), (1S, 3S)-1-methyl-1, 2, 3, 4-tetrahydro-beta-carboline-3-carboxylic acid (11) were obtained from Portulaca oleracea L., in which, compounds 4, 5, 8-11 were isolated from the plant for the first time. The structure of the compound 1 was identified using spectroscopic methods including 1D and 2D NMR, HR-ESI-TOF-MS. The compounds 1, 5-11 presented anticholinesterase activities, but the P. oleracea extract (POE) presented very low anticholinesterase activity.
[Chemical Constituents from Cynanchum paniculatum].[Pubmed:26214877]
Zhong Yao Cai. 2015 Jan;38(1):97-100.
OBJECTIVE: To investigate the chemical constituents from Gynanchum paniculatum. METHODS: The constituents were isolated and purified by silica gel, Sephadex LH-20 column chromatography, and preparative TLC. Their structures were identified on the basis of spectral data and physiochemical characteristics. RESULTS: 15 compounds were isolated from 70% ethanol extract and identified as beta-sitosterol(1), beta-daucosterin (2), mudanoside A (3), paeonolide (4), santamarin (5), paeonol(6), annobraine (7), laricircsinol (8), alpha-asarone(9), 7-angelyheliotridine(10), beta-amyrin(11), uridine(12), kaempferol-3-O-beta-D-glucopyranosyl(1-->2)alpha-L-arabinopyranoside(13), kaempferol-7-O-(4", 6"-di-p-hydroxycinnamoyl-2", 3"-diacetyl)-beta-D-glucopyranoside(14), and (2S, E)-N-[2-hydroxy-2-(4-hydroxyphenyl) ethyl] Ferulamide (15). CONCLUSION: Compounds 4, 6, 8, 11, 12 and 15 are isolated from this plant for the first time, compounds 5 and 14 are firstly isolated from the palnts of Cynanchum genus.
[Chemical constituents of Pilea cavaleriei subsp. cavaleriei].[Pubmed:23236755]
Zhongguo Zhong Yao Za Zhi. 2012 Sep;37(17):2581-4.
OBJECTIVE: To investigate chemical constituents from folk herb Pilea cavaleriei subsp. cavaleriei. METHOD: The compounds were separated and purified by silica gel, Sephadex LH-20 and the like. The structures were identified by spectral methods such as (1)H, (13)C-NMR and MS. RESULT: Seventeen compounds were isolated and identified as benzoic acid (1), 4-hydroxy benzalde-hyde (2), coumaric acid(3), protocatechuic acid (4), gallic acid (5), 4-hydroxy benzoic acid (6), 3-indole carboxaldehyde (7), 3-indole carbo-xylicacid (8), 4-methyl-(1,2,3) -triazole(9), uracil(10), nicotinamide (11), (2S,E)-N-[2-hydroxy-2-(4-hydroxy phenyl) ethyl] Ferulamide (12), (+) -dehydrovomifoliol (13), hentriantane (14), beta-sitosterol (15), palmitic acid (16), daucossterol (17) , respectively. CONCLUSION: All compounds were obtained from the genus for the first time.
Identification of (-)(E)-N-[2(S)-Hydroxy-2-(4-hydroxyphenyl) ethyl]ferulamide, a natural product isolated from Croton pullei: theoretical and experimental analysis.[Pubmed:22272139]
Int J Mol Sci. 2011;12(12):9389-403.
Ferulic acid (FA) and its derivatives (FADs) are known for a variety of biological activities, such as photo-protective agent, antioxidant, antiatherogenic and antiplasmodial activities. During structural definition of a FAD isolated from Croton pullei, the possibility of a heterologous series made this definition difficult. In this regard, computational simulations were performed using theoretical calculations at DFT level to predict Infrared (IR) and Nuclear Magnetic Resonance (NMR) data. The IR and NMR (13)C and (1)H data were compared with the theoretical calculations performed for three structural possibilities of a heterologous series. The theoretical results were compared with the experimental data through linear regression in order to define the most probable structure and showed satisfactory values.
Induction of adiponectin by natural and synthetic phenolamides in mouse and human preadipocytes and its enhancement by docosahexaenoic acid.[Pubmed:18166202]
Life Sci. 2008 Jan 30;82(5-6):290-300.
Adiponectin, the adipose-derived cytokine, plays an important role in preventing metabolic syndromes. To develop new adiponectin inducers, eight species of ferulic esters and amides, and five related compounds were synthesized and tested on the stimulation of adiponectin production in mouse 3T3-L1 and normal human preadipocytes. The Ferulamides with an aromatic ring in the N-substituent are very active in inducing adiponectin as compared with the known active compounds, curcumin, [6]-gingerol, and capsaicin, and furthermore the activities of these Ferulamides are remarkably stronger than those of the corresponding esters or the straight chain octylamide. The most active compound, N-(2-phenylethyl)Ferulamide (7), was found to activate the PPAR (peroxisome proliferator-activated receptor) gamma-RXR (retinoid X receptor) alpha heterodimeric complex in the PPRE (PPAR-responsive element)-driven luciferase reporter assay. The adiponectin production by 7 is synergistically enhanced by coaddition of a PPARgamma-specific agonist, pioglitazone (PGZ), or another PPARgamma agonist, docosahexaenoic acid (DHA), in cultured preadipocytes. The compound 7 alone did not show a statistically significant effect on the plasma adiponectin level in KK-A(y)/Ta mice, while 1% 7 in the diets significantly lowered the blood glucose and triglyceride levels and 0.3% 7 mixed with DHA oil in the diets significantly increased the adiponectin level as compared with the control. These results suggest that the present Ferulamides would be useful lead compounds in developing more potent agents for treatment of metabolic syndromes through promoting the endogenous adiponectin production, and that such an activity is possibly enhanced by the coadministration with DHA.
(2E)-N,N-dibutyl-3-(4-hydroxy-3-methoxyphenyl)acrylamide induces apoptosis and cell cycle arrest in HL-60 cells.[Pubmed:17352252]
Anticancer Res. 2007 Jan-Feb;27(1A):343-9.
BACKGROUND: Ferulic acid is one of the most ubiquitous phenolic compounds in nature, which has antioxidant and anticancer activities. However, ferulic acid derivatives, such as Ferulamide have never been reported. MATERIALS AND METHODS: (2E)-N,N-dibutyl-3-(4-hydroxy-3-methoxyphenyl)acrylamide (compound 8), a Ferulamide derivative was synthesized in our laboratory. In this study, HL-60 cells were treated with various concentrations of compound 8, and its effects on cell growth, cell cycle, apoptosis and related measurements were investigated. RESULTS: Compound 8 inhibited cell growth in a concentration- and time-dependent manner with significant cytotoxicity, and the concentration required to inhibit growth by 50% (IC50) was 8.2 microM for 24 h. The cell cycle analysis indicated that compound 8 treated cells were arrested in the G2/M-phase and followed by apoptosis. Microscopic examination showed that treatment with compound 8 displayed typical morphological features of apoptotic cells, with cell shrinking and formation of apoptotic bodies. Reverse transcription-polymerase chain reaction (RT- PCR) analysis showed a dramatic induction of CDK inhibitor p21, which inhibited the expression of cyclin B1, thereby resulting in G2/M phase arrest. After G2/M-phase arrest, cells underwent apoptosis via significant down-regulation of Bcl-2 expression. CONCLUSION: These results enhance our understanding of the mechanisms of action of compound 8-mediated anticancer effects.
Antioxidative compounds isolated from safflower (Carthamus tinctorius L.) oil cake.[Pubmed:9433760]
Chem Pharm Bull (Tokyo). 1997 Dec;45(12):1910-4.
Seven antioxidative serotonin derivatives were isolated from safflower (Carthamus tinctorius L.) oil cake. Their structures were established as N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]Ferulamide (1), N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-p-coumaramide (2), N,N'-[2,2'-(5,5'-dihydroxy-4,4'-bi-1H-indol-3,3'-yl)diethyl]- di-p-coumaramide (3), N-[2-[3'-[2-(p-coumaramido)ethyl]-5,5'-dihydroxy- 4,4'-bi-1H-indol-3-yl]ethyl]Ferulamide (4), and N,N'-[2,2'-(5,5'-dihydroxy-4,4'-bi-1H-indol-3,3'-yl)diethyl]- diFerulamide (5), N-[2-[5-(beta-D-glucosyloxy)-1H-indol-3-yl)ethyl]- p-coumaramide (6), and N-[2-[5-(beta-D-glucosyloxy)-1H-indol-3-yl)ethyl]Ferulamide (7). Antioxidative activities of the compounds were measured by the ferric thiocyanate method and the alpha,alpha-diphenyl-beta-picrylhydrazyl (DPPH) method, and compounds 1-5 were found to have relatively strong antioxidative activity.


