AtropineCAS# 51-55-8 |
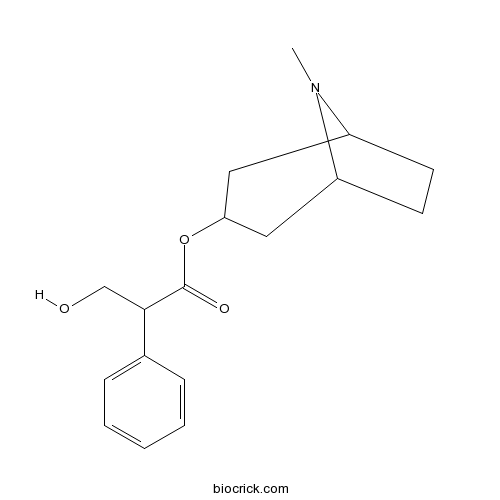
- Hyoscyamine
Catalog No.:BCN1946
CAS No.:101-31-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 51-55-8 | SDF | Download SDF |
| PubChem ID | 3661 | Appearance | Powder |
| Formula | C17H23NO3 | M.Wt | 289.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Tropine tropate; DL-Hyoscyamine | ||
| Solubility | DMSO : ≥ 96.6 mg/mL (333.83 mM) H2O : 2.9 mg/mL (10.02 mM; Need ultrasonic and warming) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (8-methyl-8-azabicyclo[3.2.1]octan-3-yl) 3-hydroxy-2-phenylpropanoate | ||
| SMILES | CN1C2CCC1CC(C2)OC(=O)C(CO)C3=CC=CC=C3 | ||
| Standard InChIKey | RKUNBYITZUJHSG-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Atropine is a competitive antagonist of the muscarinic acetylcholine receptors (acetylcholine being the main neurotransmitter used by the parasympathetic nervous system), used to treat certain types of nerve agent and pesticide poisonings, some types of slow heart rate, and to decrease saliva production during surgery. |
| Targets | AChR |
| In vitro | Use of atropine penalization to treat amblyopia in UK orthoptic practice.[Pubmed: 25427306]J Pediatr Ophthalmol Strabismus. 2014 Nov-Dec;51(6):363-9.To compare clinical practice patterns regarding Atropine penalization use by UK orthoptists to the current evidence base and identify any existing barriers against use of AP as first-line treatment. |
| In vivo | Use of atropine-diphenoxylate compared with hyoscyamine to decrease rates of irinotecan-related cholinergic syndrome.[Pubmed: 25839059]J Community Support Oncol. 2015 Jan;13(1):3-7.To compare the incidence of cholinergic syndrome with irinotecan using Atropine-diphenoxylate or hyoscyamine as premedication. Atropine first is safer than conventional atropine administration in older people undergoing dobutamine stress echocardiography.[Pubmed: 24906705]Ther Adv Cardiovasc Dis. 2014 Oct;8(5):176-84.Early injection of Atropine during dobutamine stress echocardiography (DSE) has been demonstrated in retrospective analyses to reduce the duration and dose of dobutamine infusion, while preserving a similar diagnostic accuracy with a lower incidence of adverse effects. This study explores the safety of using Atropine as a start drug before dobutamine infusion (ADSE protocol) in comparison with the conventional protocol (DASE protocol) in older patients undergoing DSE for ischemia evaluation.
|

Atropine Dilution Calculator

Atropine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4554 mL | 17.2771 mL | 34.5543 mL | 69.1085 mL | 86.3856 mL |
| 5 mM | 0.6911 mL | 3.4554 mL | 6.9109 mL | 13.8217 mL | 17.2771 mL |
| 10 mM | 0.3455 mL | 1.7277 mL | 3.4554 mL | 6.9109 mL | 8.6386 mL |
| 50 mM | 0.0691 mL | 0.3455 mL | 0.6911 mL | 1.3822 mL | 1.7277 mL |
| 100 mM | 0.0346 mL | 0.1728 mL | 0.3455 mL | 0.6911 mL | 0.8639 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Atropine is a medication used to treat certain types of nerve agent and pesticide poisonings, some types of slow heart rate, and to decrease saliva production during surgery.
References:
[1]. Montano N, et al. Central vagotonic effects of atropine modulate spectral oscillations of sympathetic nerve activity. Circulation, 1998, 98(14):1394-1399
[2]. Debas HT, et al. After vagotomy atropine suppresses gastrin release by food. Gastroenterology. 1976 Jun;70(6):1082-4.
- Propylthiouracil
Catalog No.:BCC4931
CAS No.:51-52-5
- L-Thyroxine
Catalog No.:BCC4917
CAS No.:51-48-9
- Histamine
Catalog No.:BCN2188
CAS No.:51-45-6
- Adrenaline
Catalog No.:BCN2191
CAS No.:51-43-4
- Epinephrine Bitartrate
Catalog No.:BCC4348
CAS No.:51-42-3
- Norepinephrine
Catalog No.:BCN2206
CAS No.:51-41-2
- H-Hyp-OH
Catalog No.:BCC3250
CAS No.:51-35-4
- Scopolamine
Catalog No.:BCN5045
CAS No.:51-34-3
- Isoprenaline HCl
Catalog No.:BCC4328
CAS No.:51-30-9
- Tiratricol
Catalog No.:BCC4738
CAS No.:51-24-1
- Fluorouracil (Adrucil)
Catalog No.:BCC2135
CAS No.:51-21-8
- Benzimidazole
Catalog No.:BCC8847
CAS No.:51-17-2
- Homatropine Bromide
Catalog No.:BCC4570
CAS No.:51-56-9
- D-Amphetamine sulfate
Catalog No.:BCC5942
CAS No.:51-63-8
- 4'-Methoxyacetanilide
Catalog No.:BCC8711
CAS No.:51-66-1
- Tyramine
Catalog No.:BCN6776
CAS No.:51-67-2
- Carbamoylcholine chloride
Catalog No.:BCC7492
CAS No.:51-83-2
- (2-Acetoxyethyl)trimethylammonium
Catalog No.:BCN1743
CAS No.:51-84-3
- Tetramethylammonium
Catalog No.:BCN1816
CAS No.:51-92-3
- Trachelanthine
Catalog No.:BCN2042
CAS No.:510-19-0
- Voacangine
Catalog No.:BCN3224
CAS No.:510-22-5
- Belladonnine
Catalog No.:BCN1892
CAS No.:510-25-8
- Echinocystic acid
Catalog No.:BCN5628
CAS No.:510-30-5
- AMI-193
Catalog No.:BCC6679
CAS No.:510-74-7
Use of atropine-diphenoxylate compared with hyoscyamine to decrease rates of irinotecan-related cholinergic syndrome.[Pubmed:25839059]
J Community Support Oncol. 2015 Jan;13(1):3-7.
BACKGROUND: Cholinergic syndrome is a well established acute adverse reaction associated with irinotecan. Cholinergic side effects can be ameliorated or prevented with anticholinergic agents. To date, no formal studies have compared Atropine-diphenoxylate and hyoscyamine as premedications for prophylaxis of the cholinergic syndrome with irinotecan infusion. OBJECTIVES: To compare the incidence of cholinergic syndrome with irinotecan using Atropine-diphenoxylate or hyoscyamine as premedication. METHODS: We conducted a retrospective, single-center, nonrandomized, cohort study of adult patients treated with Atropine-diphenoxylate or hyoscyamine as premedication before receiving irinotecan. For all irinotecan infusions, intravenous Atropine was administered for patients experiencing any cholinergic reaction. RESULTS: A total of 532 irinotecan cycles (354 cycles for Atropine-diphenoxylate group; 178 cycles for hyoscyamine group) were analyzed in 80 patients. Overall incidence of cholinergic syndrome did not differ between Atropine-diphenoxylate (8.2%) and hyoscyamine (9.0%) groups (P = .76). The incidence of cholinergic syndrome after the poundrst cycle of irinotecan was similar between the 2 arms, Atropine-diphenoxylate (14.6%) and hyoscyamine (10.7%), with P = .74. The most common cholinergic symptoms documented were abdominal pain or cramping, and diarrhea. LIMITATIONS: This study was subjected to vulnerabilities to bias and random error because of its observational retrospective design and small number of participants. CONCLUSIONS: Lack of difference in the incidence of cholinergic syndrome observed in irinotecan-treated patients suggests Atropinediphenoxylate and hyoscyamine may both be effective prophylactic options. The findings support the need for a larger, randomized study to assess and compare these agents with other potential premedications such as scopolamine and Atropine in prevention of irinotecan-related cholinergic syndrome.
Atropine first is safer than conventional atropine administration in older people undergoing dobutamine stress echocardiography.[Pubmed:24906705]
Ther Adv Cardiovasc Dis. 2014 Oct;8(5):176-84.
OBJECTIVE: Early injection of Atropine during dobutamine stress echocardiography (DSE) has been demonstrated in retrospective analyses to reduce the duration and dose of dobutamine infusion, while preserving a similar diagnostic accuracy with a lower incidence of adverse effects. This study explores the safety of using Atropine as a start drug before dobutamine infusion (ADSE protocol) in comparison with the conventional protocol (DASE protocol) in older patients undergoing DSE for ischemia evaluation. METHODS: One hundred consecutive older patients were prospectively enrolled. When eligible, they were randomly assigned to undergo either the DASE protocol (group A, 50 patients) or the ADSE protocol (group B, 50 patients) when Atropine (1.0 mg) was first administered 3 min before dobutamine infusion followed by 0.5 mg increments (maximum 1.0 mg) thereafter. Patients were monitored for adverse drug effects. Test duration was calculated. RESULTS: The mean age of the whole study cohort was 67.8+/-4.3 years and 58 (58%) were men. Patients in group A had longer test duration (21.8+/-1.3 versus 13.7+/-0.77 min, p<0.001) and higher mean dobutamine infusion rate (39+/-8.2 versus 28.2+/-9.5 mug/kg/min, p<0.001). The two groups received a similar total dose of Atropine. Group A patients showed significantly higher incidence of extrasystoles, nonsustained ventricular tachycardia and severe hypotension (p<0.05). CONCLUSION: In older patients undergoing DSE, using Atropine as a start drug, that is, adopting the ADSE protocol, is associated with shorter test duration, lower mean dobutamine infusion rate and consequently fewer adverse effects.
Use of atropine penalization to treat amblyopia in UK orthoptic practice.[Pubmed:25427306]
J Pediatr Ophthalmol Strabismus. 2014 Nov-Dec;51(6):363-9.
PURPOSE: To compare clinical practice patterns regarding Atropine penalization use by UK orthoptists to the current evidence base and identify any existing barriers against use of AP as first-line treatment. METHODS: An online survey was designed to assess current practice patterns of UK orthoptists using Atropine penalization. They were asked to identify issues limiting their use of Atropine penalization and give opinions on its effectiveness compared to occlusion. Descriptive statistics and content analysis were applied to the results. RESULTS: Responses were obtained from 151 orthoptists throughout the United Kingdom. The main perceived barriers to use of Atropine penalization were inability to prescribe Atropine and supply difficulties. However, respondents also did not consider Atropine penalization as effective as occlusion in treating amblyopia, contrary to recent research findings. Patient selection criteria and treatment administration largely follow current evidence. More orthoptists use Atropine penalization as first-line treatment than previously reported. CONCLUSIONS: Practitioners tend to closely follow the current evidence base when using Atropine penalization, but reluctance in offering it as first-line treatment or providing a choice for parents between occlusion and Atropine still remains. This may result from concerns regarding Atropine's general efficacy, side effects, and risk of reverse amblyopia. Alternatively, as demonstrated in other areas of medicine, it may reflect the inherent delay of research findings translating to clinical practice changes.


