TyramineCAS# 51-67-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 51-67-2 | SDF | Download SDF |
| PubChem ID | 5610 | Appearance | Powder |
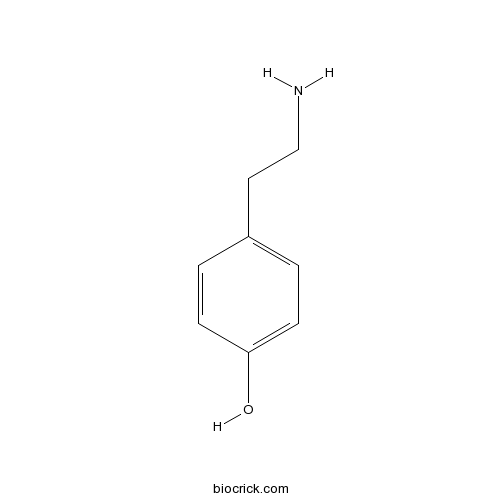
| Formula | C8H11NO | M.Wt | 137.18 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-(2-aminoethyl)phenol | ||
| SMILES | C1=CC(=CC=C1CCN)O | ||
| Standard InChIKey | DZGWFCGJZKJUFP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H11NO/c9-6-5-7-1-3-8(10)4-2-7/h1-4,10H,5-6,9H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tyramine is a major mutagen precursor in soy sauce, being convertible to a mutagen by nitrite; it is the biological precursor of octopamine, both compounds are independent neurotransmitters, acting through various G-protein coupled receptors, they are antagonistic modulators of behavior and metabolism. |
| Targets | G-protein coupled receptors |
| In vivo | Tyramine Functions independently of octopamine in the Caenorhabditis elegans nervous system.[Reference: WebLink]Neuron, 2005 ,46 (2) :247-60.Octopamine biosynthesis requires tyrosine decarboxylase to convert tyrosine into Tyramine and Tyramine beta-hydroxylase to convert Tyramine into octopamine. |
| Kinase Assay | Tyramine and octopamine: antagonistic modulators of behavior and metabolism.[Pubmed: 12942511 ]Arch Insect Biochem Physiol. 2003 Sep;54(1):1-13.The phenolamines Tyramine and octopamine are decarboxylation products of the amino acid tyrosine. |
| Structure Identification | Gan. 1984 Jan;75(1):1-3.Tyramine is a major mutagen precursor in soy sauce, being convertible to a mutagen by nitrite.[Pubmed: 6373470]Tyramine was identified as a new mutagen precursor in Japanese soy sauce, becoming mutagenic after treatment with nitrite under acidic conditions. The mutagenic compound was identified as 4-(2-aminoethyl)-6-diazo-2,4- cyclohexadienone , and its specific mutagenic activity was 112 revertants/micrograms towards Salmonella typhimurium TA100 without S9 mix. |

Tyramine Dilution Calculator

Tyramine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.2897 mL | 36.4485 mL | 72.8969 mL | 145.7938 mL | 182.2423 mL |
| 5 mM | 1.4579 mL | 7.2897 mL | 14.5794 mL | 29.1588 mL | 36.4485 mL |
| 10 mM | 0.729 mL | 3.6448 mL | 7.2897 mL | 14.5794 mL | 18.2242 mL |
| 50 mM | 0.1458 mL | 0.729 mL | 1.4579 mL | 2.9159 mL | 3.6448 mL |
| 100 mM | 0.0729 mL | 0.3645 mL | 0.729 mL | 1.4579 mL | 1.8224 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4'-Methoxyacetanilide
Catalog No.:BCC8711
CAS No.:51-66-1
- D-Amphetamine sulfate
Catalog No.:BCC5942
CAS No.:51-63-8
- Homatropine Bromide
Catalog No.:BCC4570
CAS No.:51-56-9
- Atropine
Catalog No.:BCN5639
CAS No.:51-55-8
- Propylthiouracil
Catalog No.:BCC4931
CAS No.:51-52-5
- L-Thyroxine
Catalog No.:BCC4917
CAS No.:51-48-9
- Histamine
Catalog No.:BCN2188
CAS No.:51-45-6
- Adrenaline
Catalog No.:BCN2191
CAS No.:51-43-4
- Epinephrine Bitartrate
Catalog No.:BCC4348
CAS No.:51-42-3
- Norepinephrine
Catalog No.:BCN2206
CAS No.:51-41-2
- H-Hyp-OH
Catalog No.:BCC3250
CAS No.:51-35-4
- Scopolamine
Catalog No.:BCN5045
CAS No.:51-34-3
- Carbamoylcholine chloride
Catalog No.:BCC7492
CAS No.:51-83-2
- (2-Acetoxyethyl)trimethylammonium
Catalog No.:BCN1743
CAS No.:51-84-3
- Tetramethylammonium
Catalog No.:BCN1816
CAS No.:51-92-3
- Trachelanthine
Catalog No.:BCN2042
CAS No.:510-19-0
- Voacangine
Catalog No.:BCN3224
CAS No.:510-22-5
- Belladonnine
Catalog No.:BCN1892
CAS No.:510-25-8
- Echinocystic acid
Catalog No.:BCN5628
CAS No.:510-30-5
- AMI-193
Catalog No.:BCC6679
CAS No.:510-74-7
- Galanthaminone
Catalog No.:BCN2867
CAS No.:510-77-0
- Minecoside
Catalog No.:BCN5627
CAS No.:51005-44-8
- 6''-O-Malonylgenistin
Catalog No.:BCN2772
CAS No.:51011-05-3
- Isocorynoxeine
Catalog No.:BCN5003
CAS No.:51014-29-0
Tyramine and octopamine: antagonistic modulators of behavior and metabolism.[Pubmed:12942511]
Arch Insect Biochem Physiol. 2003 Sep;54(1):1-13.
The phenolamines Tyramine and octopamine are decarboxylation products of the amino acid tyrosine. Although Tyramine is the biological precursor of octopamine, both compounds are independent neurotransmitters, acting through various G-protein coupled receptors. Especially, octopamine modulates a plethora of behaviors, peripheral and sense organs. Both compounds are believed to be homologues of their vertebrate counterparts adrenaline and noradrenaline. They modulate behaviors and organs in a coordinated way, which allows the insects to respond to external stimuli with a fine tuned adequate response. As these two phenolamines are the only biogenic amines whose physiological significance is restricted to invertebrates, the attention of pharmacologists was focused on the corresponding receptors, which are still believed to represent promising targets for new insecticides. Recent progress made on all levels of octopamine/Tyramine research enabled us to better understand the molecular events underlying the control of complex behaviors.
Tyramine is a major mutagen precursor in soy sauce, being convertible to a mutagen by nitrite.[Pubmed:6373470]
Gan. 1984 Jan;75(1):1-3.
Tyramine was identified as a new mutagen precursor in Japanese soy sauce, becoming mutagenic after treatment with nitrite under acidic conditions. The mutagenic compound was identified as 4-(2-aminoethyl)-6-diazo-2,4- cyclohexadienone , and its specific mutagenic activity was 112 revertants/micrograms towards Salmonella typhimurium TA100 without S9 mix.


