1-BenzhydrylpiperazineCAS# 841-77-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 841-77-0 | SDF | Download SDF |
| PubChem ID | 70048 | Appearance | Powder |
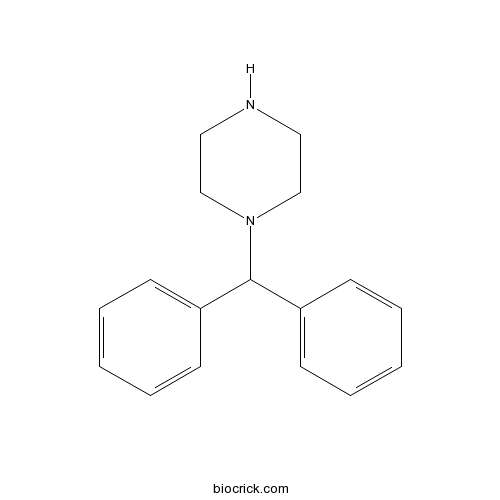
| Formula | C17H20N2 | M.Wt | 252.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-benzhydrylpiperazine | ||
| SMILES | C1CN(CCN1)C(C2=CC=CC=C2)C3=CC=CC=C3 | ||
| Standard InChIKey | NWVNXDKZIQLBNM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H20N2/c1-3-7-15(8-4-1)17(16-9-5-2-6-10-16)19-13-11-18-12-14-19/h1-10,17-18H,11-14H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

1-Benzhydrylpiperazine Dilution Calculator

1-Benzhydrylpiperazine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.962 mL | 19.8098 mL | 39.6197 mL | 79.2393 mL | 99.0491 mL |
| 5 mM | 0.7924 mL | 3.962 mL | 7.9239 mL | 15.8479 mL | 19.8098 mL |
| 10 mM | 0.3962 mL | 1.981 mL | 3.962 mL | 7.9239 mL | 9.9049 mL |
| 50 mM | 0.0792 mL | 0.3962 mL | 0.7924 mL | 1.5848 mL | 1.981 mL |
| 100 mM | 0.0396 mL | 0.1981 mL | 0.3962 mL | 0.7924 mL | 0.9905 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Roquinimex
Catalog No.:BCC5355
CAS No.:84088-42-6
- Lamotrigine
Catalog No.:BCC5051
CAS No.:84057-84-1
- Helioxanthin 8-1
Catalog No.:BCC5415
CAS No.:840529-13-7
- Fmoc-N-Me-Val-OH
Catalog No.:BCC3358
CAS No.:84000-11-3
- Fmoc-N-Me-Ala-OH
Catalog No.:BCC3210
CAS No.:84000-07-7
- Xanthoxyletin
Catalog No.:BCN6579
CAS No.:84-99-1
- Vitamin K1
Catalog No.:BCN2209
CAS No.:84-80-0
- Lapachol
Catalog No.:BCN4391
CAS No.:84-79-7
- Dibutyl Phthalate
Catalog No.:BCC8411
CAS No.:84-74-2
- Diisobutyl phthalate
Catalog No.:BCN7148
CAS No.:84-69-5
- Anthraquinone
Catalog No.:BCC8832
CAS No.:84-65-1
- Anthraflavic acid
Catalog No.:BCC8831
CAS No.:84-60-6
- Wilforlide A
Catalog No.:BCN4383
CAS No.:84104-71-2
- Wilforlide A acetate
Catalog No.:BCN4384
CAS No.:84104-80-3
- Triptotriterpenic acid A
Catalog No.:BCN6780
CAS No.:84108-17-8
- R406 (free base)
Catalog No.:BCC2553
CAS No.:841290-80-0
- R406
Catalog No.:BCC3876
CAS No.:841290-81-1
- 6-Fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride
Catalog No.:BCC8772
CAS No.:84163-13-3
- Ac-Glu(OtBu)-OH
Catalog No.:BCC2921
CAS No.:84192-88-1
- 7 8-Dihydroxy-4-Phenylcoumarin
Catalog No.:BCC8289
CAS No.:842-01-3
- Sudan I
Catalog No.:BCN8378
CAS No.:842-07-9
- Cyclosomatostatin
Catalog No.:BCC7693
CAS No.:84211-54-1
- Canagliflozin
Catalog No.:BCC3696
CAS No.:842133-18-0
- AKT Kinase Inhibitor
Catalog No.:BCC1335
CAS No.:842148-40-7
Synthesis, biological evaluation and molecular modeling studies of phenyl-/benzhydrylpiperazine derivatives as potential MAO inhibitors.[Pubmed:29421700]
Bioorg Chem. 2018 Apr;77:252-262.
Monoamine oxidase inhibitors (MAOIs) are potential drug candidates for the treatment of various neurological disorders like Parkinson's disease, Alzheimer's disease and depression. In the present study, two series of 4-substituted phenylpiperazine and 1-Benzhydrylpiperazine (1-21) derivatives were synthesized and screened for their MAO-A and MAO-B inhibitory activity using Amplex Red assay. Most of the synthesized compounds were found selective for MAO-B isoform except compounds 3, 7, 8, 9 and 13 (MAO-A selective) while compound 11 was non-selective. In the current series, compound 12 showed most potent MAO-B inhibitor activity with IC50 value of 80nM and compound 7 was found to be most potent MAO-A inhibitor with IC50 value of 120nM and both the compounds were found reversible inhibitors. Compound 8 was found most selective MAO-A inhibitor while compound 20 was found most selective inhibitor for MAO-B isoform. In the cytotoxicity evaluation, all the compounds were found non-toxic to SH-SY5Y and IMR-32 cells at 25microM concentration. In the ROS studies, compound 8 (MAO-A inhibitor) reduced the ROS level by 51.2% while compound 13 reduced the ROS level by 61.81%. In the molecular dynamic simulation studies for 30ns, compound 12 was found quite stable in the active cavity of MAO-B. Thus, it can be concluded that phenyl- and 1-Benzhydrylpiperazine derivatives are promising MAO inhibitors and can act as a lead to design potent, and selective MAO inhibitors for the treatment of various neurological disorders.
Novel Sulfamide-Containing Compounds as Selective Carbonic Anhydrase I Inhibitors.[Pubmed:28672822]
Molecules. 2017 Jun 24;22(7). pii: molecules22071049.
The development of isoform selective inhibitors of the carbonic anhydrase (CA; EC 4.2.1.1) enzymes represents the key approach for the successful development of druggable small molecules. Herein we report a series of new benzenesulfamide derivatives (-NH-SO(2)NH(2)) bearing the 1-Benzhydrylpiperazine tail and connected by means of a beta-alanyl or nipecotyl spacer. All compounds 6a-l were investigated in vitro for their ability to inhibit the physiological relevant human (h) CA isoforms such as I, II, IV and IX. Molecular modeling provided further structural support to enzyme inhibition data and structure-activity relationship. In conclusion the hCA I resulted the most inhibited isoform, whereas all the remaining ones showed different inhibition profiles.
Synthesis and in vitro antiproliferative activity of novel 1-benzhydrylpiperazine derivatives against human cancer cell lines.[Pubmed:18973966]
Eur J Med Chem. 2009 Mar;44(3):1223-9.
In order to explore the antiproliferative effect associated with the piperazine framework, several 1-Benzhydrylpiperazine derivatives 8(a-d), 9(a-d) and 10(a-h) were synthesized. Variation in the functional group at N-terminal of the piperazine led to three sets of compounds, bearing the sulfonyl, amide and thiourea, respectively. Their chemical structures were confirmed by (1)H NMR, LCMS, IR and elemental analysis. The antiproliferative effect of the compounds were evaluated in vitro using the MTT colorimetric method against one normal cell line (NF-103 skin fibroblast cells) and four human cancer cell lines (MCF-7 breast carcinoma cell line, HepG-2 hepatocellular carcinoma cell line, HeLa cervix carcinoma cell line and HT-29 colon carcinoma cell line) for the time period of 24 h. Among the series, four compounds exhibited interesting growth inhibitory effects against all four cell lines.
Design, synthesis and biological evaluation of piperazine analogues as CB1 cannabinoid receptor ligands.[Pubmed:18243711]
Bioorg Med Chem. 2008 Apr 1;16(7):4035-51.
After the CB1 receptor antagonist SR141716 (rimonabant) was previously reported to modulate food intake, CB1 antagonism has been considered as a new therapeutic target for the treatment of obesity. Several series of urea, carbamate, amide, sulfonamide and oxalamide derivatives based on 1-Benzhydrylpiperazine scaffold were synthesized and tested for CB1 receptor binding affinity. The SAR studies to optimize the CB1 binding affinity led to the potent urea derivatives. After the additional SAR studies to optimize the substituents of diphenyl rings, the combination of 2-chlorophenyl and 4-chlorophenyl turned out to be the most potent scaffold. The CB2 binding affinity assay as well as functional assay was also conducted on these compounds. Herein we wish to introduce several novel CB1 antagonists with IC(50) values less than 100 nM for the CB1 receptor binding.
1-Benzhydryl-4-(4-chloro-phenyl-sulfonyl)piperazine.[Pubmed:21201390]
Acta Crystallogr Sect E Struct Rep Online. 2008 Jan 4;64(Pt 2):o358.
The title compound, C(23)H(23)ClN(2)O(2)S, was synthesized by the nucleophilic substitution of 1-Benzhydrylpiperazine with 4-chloro-phenyl-sulfonyl chloride. The piperazine ring is in a chair conformation. The geometry around the S atom is that of a distorted tetra-hedron. There is a large range of bond angles around the piperazine N atoms. The dihedral angle between the least-squares plane (p1) defined by the four coplanar C atoms of the piperazine ring and the benzene ring is 81.6 (1) degrees . The dihedral angles between p1 and the phenyl rings are 76.2 (1) and 72.9 (2) degrees . The two phenyl rings make a dihedral angle of 65.9 (1) degrees . Intramolecular C-Hcdots, three dots, centeredO hydrogen bonds are present.
[Piperazines as model substrate for oxidations].[Pubmed:17069419]
Pharmazie. 2006 Oct;61(10):815-22.
Piperazines as model substrate for oxidations Piperazine derivatives when being oxidized by mercury-EDTA behave unusually. Due to the reactive cyclic enediamine intermediates as aza-analogous reductones and to the carbonyl compounds resulting from dehydrogenation in the side chain, there exists a high tendency of polymerization. 1-Benzylpiperazines 5a-d generate the piperazine-2,3-diones 8a-d in medium yields. From 1-Benzhydrylpiperazine 11 results a mixture of piperazine-2,3-dione 12 and piperazine-3-on 13. The 1,4-bis-substituted piperazines react more differently because of the symmetry and the preferred direction of the dehydrogenation into the cycle. Thus, from 15 and 19 the diones 16 and 20, respectively, were available in very good yields. A mechanism for the reactions is proposed.


