AM251Potent CB1 antagonist CAS# 183232-66-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 183232-66-8 | SDF | Download SDF |
| PubChem ID | 2125 | Appearance | Powder |
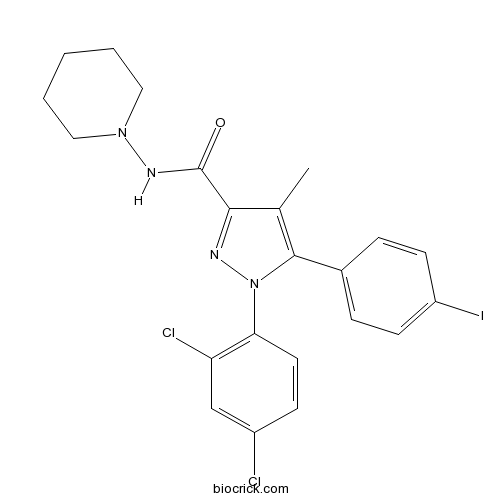
| Formula | C22H21Cl2IN4O | M.Wt | 555.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 25 mg/mL (45.03 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-piperidin-1-ylpyrazole-3-carboxamide | ||
| SMILES | CC1=C(N(N=C1C(=O)NN2CCCCC2)C3=C(C=C(C=C3)Cl)Cl)C4=CC=C(C=C4)I | ||
| Standard InChIKey | BUZAJRPLUGXRAB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H21Cl2IN4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-7-16(23)13-18(19)24)21(14)15-5-8-17(25)9-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent CB1 receptor antagonist (IC50 = 8 nM, Ki = 7.49 nM) that displays 306-fold selectivity over CB2 receptors. Also potent GPR55 agonist (EC50 = 39 nM). Also available in a fluorescent form. |

AM251 Dilution Calculator

AM251 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.801 mL | 9.0051 mL | 18.0102 mL | 36.0205 mL | 45.0256 mL |
| 5 mM | 0.3602 mL | 1.801 mL | 3.602 mL | 7.2041 mL | 9.0051 mL |
| 10 mM | 0.1801 mL | 0.9005 mL | 1.801 mL | 3.602 mL | 4.5026 mL |
| 50 mM | 0.036 mL | 0.1801 mL | 0.3602 mL | 0.7204 mL | 0.9005 mL |
| 100 mM | 0.018 mL | 0.0901 mL | 0.1801 mL | 0.3602 mL | 0.4503 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AM521 is a potent cannabinoid 1 (CB1) receptor antagonist with IC50 of 8 nM and Ki of 7.49 nM.
The cannabinoid receptor (CB1 and CB2) is a member of G-protein coupled receptor which plays a significant role in physiologic processes such as cognitive and immune functions.
AM251 inhibited the coupling of cannabinoid 1 receptor agonists and antagonists onto the rat brain membranes [1]. In hippocampus, 2 μm AM 251 reduced the endocannabinoids inhibitory effects on GABA release [2]. 1 μm AM 251 inhibited endocannabinoid-signaled depression of interneuron firing [3]. AM251 reduced neuronal excitability and inhibited excitatory and inhibitory transmitter release via inhibition of voltage-dependent Na+ channels [4].
AM251 caused sustained anorectic effect in rat for obesity treatment [5]. AM251 may also disrupt the training – induced increase of hippocampus cannabinoids level that promotes memory consolidation [6].
References:
[1] Lan, R, Q. Liu, P. Fan, S. Lin, S. R. Fernando, D. McCallion, R. Pertwee & A. Makriyannis: Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J. Med. Chem. 1999, 42, 769–776.
[2] Willow, M. & W. A. Catterall: Inhibition of binding of [3H]batrachotoxin A 20-α-benzoate to sodium channels by the anticonvulsant drugs diphenylhydantoin and carbamazepine. Mol. Pharmacol. 1982, 22, 627–635.
[3] Kreitzer, A. C., A. G. Carter & W. G. Regehr: Inhibition of interneuron firing extends the spread of endocannabinoid signaling in the cerebellum. Neuron 2002, 34, 787–796.
[4] Liao C, Zheng J, David LS, Nicholson RA. Inhibition of voltage-sensitive sodium channels by the cannabinoid 1 receptor antagonist AM 251 in mammalian brain. Basic Clin Pharmacol Toxicol. 2004 Feb;94(2):73-8.
[5] Chambers AP, Sharkey KA, Koopmans HS. Cannabinoid (CB)1 receptor antagonist, AM 251, causes a sustained reduction of daily food intake in the rat. Physiol Behav. 2004 Oct 15;82(5):863-9.
[6] de Oliveira Alvares L, de Oliveira LF, Camboim C, Diehl F, Genro BP, Lanziotti VB, Quillfeldt JA. Amnestic effect of intrahippocampal AM251, a CB1-selective blocker, in the inhibitory avoidance, but not in the open field habituation task, in rats. Neurobiol Learn Mem. 2005 Mar;83(2):119-24.
- 1,7-Dihydroxy-2,3-methylenedioxyxanthone
Catalog No.:BCN7543
CAS No.:183210-63-1
- Tipiracil hydrochloride
Catalog No.:BCC2001
CAS No.:183204-72-0
- Guanylin (human)
Catalog No.:BCC7204
CAS No.:183200-12-6
- Cabazitaxel
Catalog No.:BCC4966
CAS No.:183133-96-2
- Fabiatrin
Catalog No.:BCN2920
CAS No.:18309-73-4
- 3,4-O-Isopropylidene shikimic acid
Catalog No.:BCN1147
CAS No.:183075-03-8
- Didrovaltrate
Catalog No.:BCN7124
CAS No.:18296-45-2
- Valepotriate
Catalog No.:BCN2351
CAS No.:18296-44-1
- Dibritannilactone B
Catalog No.:BCN7776
CAS No.:1829580-18-8
- (9Z,12Z)-N-Benzyloctadeca-9,12-dienamide
Catalog No.:BCN1518
CAS No.:18286-71-0
- Thalidezine
Catalog No.:BCN7763
CAS No.:18251-36-0
- SB 225002
Catalog No.:BCC8077
CAS No.:182498-32-4
- 2-Acetoxy-3-deacetoxycaesaldekarin E
Catalog No.:BCN7476
CAS No.:18326-06-2
- 5-(1-Piperazinyl)benzofuran-2-carboxamide
Catalog No.:BCC8717
CAS No.:183288-46-2
- Erlotinib Hydrochloride
Catalog No.:BCC3645
CAS No.:183319-69-9
- OSI-420
Catalog No.:BCC4472
CAS No.:183320-51-6
- Erlotinib
Catalog No.:BCC1557
CAS No.:183321-74-6
- CPPG
Catalog No.:BCC6872
CAS No.:183364-82-1
- Apicidin
Catalog No.:BCC3599
CAS No.:183506-66-3
- Cleroindicin A
Catalog No.:BCC8916
CAS No.:176598-06-4
- CYN 154806
Catalog No.:BCC5823
CAS No.:183658-72-2
- Penthiopyrad
Catalog No.:BCC8072
CAS No.:183675-82-3
- 1,2,3,4,5,6-Hexabromocyclohexane
Catalog No.:BCC2437
CAS No.:1837-91-8
- MRS 1220
Catalog No.:BCC6972
CAS No.:183721-15-5
Prevention of drug priming- and cue-induced reinstatement of MDMA-seeking behaviors by the CB1 cannabinoid receptor antagonist AM251.[Pubmed:26796595]
Drug Alcohol Depend. 2016 Mar 1;160:76-81.
BACKGROUND: 3,4-Methylenedioxymethamphetamine (MDMA), a methamphetamine (METH) derivative, exhibits METH-like actions at monoamine transporters and positive reinforcing effects in rodents and primates. The purposes of the present study were to determine whether cross-reinstatement would be observed between MDMA and METH and if the cannabinoid receptor, a receptor known to play critical roles in the brain reward system, could modulate MDMA craving. METHODS: Rats were trained to press a lever for intravenous MDMA (0.3mg/infusion) or METH (0.02mg/infusion) infusions under a fixed ratio 1 schedule paired with drug-associated cues (light and tone). Following drug self-administration acquisition training, rats underwent extinction training (an infusion of saline). Reinstatement tests were performed once the extinction criteria were achieved. RESULTS: In MDMA-trained rats, the MDMA-priming injection (3.2mg/kg, i.p.) or re-exposure to MDMA-associated cues reinstated MDMA-seeking behavior. Additionally, a priming injection of METH (1.0mg/kg, i.p.) also reinstated MDMA-seeking behavior. In contrast, none of the MDMA doses reinstated METH-seeking behavior in the METH-trained rats. The CB1 cannabinoid receptor antagonist AM251 markedly attenuated the MDMA-seeking behaviors induced by MDMA-priming injection or re-exposure to MDMA-associated cues in a dose-dependent manner. CONCLUSIONS: These findings show that MDMA has obvious addictive potential for reinstating drug-seeking behavior and that METH can be an effective stimulus for reinstating MDMA-seeking behaviors. Furthermore, based on the attenuating effect of AM251 in the reinstatement of MDMA-seeking behaviors, drugs that suppress CB1 receptors may be used in treatment of MDMA dependence.
AM251 Suppresses Epithelial-Mesenchymal Transition of Renal Tubular Epithelial Cells.[Pubmed:27936102]
PLoS One. 2016 Dec 9;11(12):e0167848.
Epithelial-mesenchymal transition (EMT) of renal tubular epithelial cells is one of the causative mechanisms of kidney fibrosis. In our study, we screened lipophilic compounds using a lipid library including approximately 200 lipids to identify those that suppressed EMT induced by a transforming growth factor (TGF)-beta1 stimulus. Initial screening was performed with the immortalized HK-2 renal tubule epithelial cell line. The most promising compounds were further tested in RPTEC primary renal tubule epithelial cells. We found that the synthetic lipid AM251 suppressed two hallmark events associated with EMT, the upregulation of collagen 1A1 (COL1A1) and downregulation of E-cadherin. Though AM251 is known to act as an antagonist for the cannabinoid receptor type 1 (CB1) and an agonist for the G protein-coupled receptor 55 (GRP55), the suppression of EMT by AM251 was not mediated through either receptor. Microarray analyses revealed that AM251 inhibited induction of several EMT transcription factors such as SNAIL1, which is the key inducer of EMT, and the AP-1 transcription factors FOSB and JUNB. Activation of SMAD2/3 and p38 mitogen-activated protein kinase (MAPK) was inhibited by AM251, with greater inhibition of the latter, indicating that AM251 acted upstream of SMAD/p38 MAPK in the TGF-beta signaling pathway. Our findings regarding the effects of AM251 on the TGF-beta signaling pathway may inform development of a novel therapeutic agent suppressing EMT, thus preventing kidney fibrosis.
Interactive effects of AM251 and baclofen on synaptic plasticity in the rat dentate gyrus.[Pubmed:27663967]
Brain Res. 2016 Nov 15;1651:53-60.
Long-term potentiation (LTP), a form of synaptic plasticity, is considered to be a critical cellular mechanism that underlies learning and memory. Cannabinoid CB1 and metabotropic GABAB receptors display similar pharmacological effects and co-localize in certain brain regions. In this study, we examined the effects of co-administration of the CB1 and GABAB antagonists AM251 and baclofen, respectively, on LTP induction in the rat dentate gyrus (DG). Male Wistar rats were anesthetized with urethane. A stimulating electrode was placed in the lateral perforant path (PP), and a bipolar recording electrode was inserted into the DG until maximal field excitatory postsynaptic potentials (fEPSPs) were observed. LTP was induced in the hippocampal area by high-frequency stimulation (HFS) of the PP. fEPSPs and population spikes (PS) were recorded at 5, 30, and 60min after HFS in order to measure changes in the synaptic responses of DG neurons. Our results showed that HFS coupled with administration of AM251 and baclofen increased both PS amplitude and fEPSP slope. Furthermore, co-administration of AM251 and baclofen elicited greater increases in PS amplitude and fEPSP slope. The results of the present study suggest that CB1 receptor activation in the hippocampus mainly modifies synapses onto GABAergic interneurons located in the DG. Our results further suggest that, when AM251 and baclofen are administered simultaneously, AM251 can alter GABA release and thereby augment LTP through GABAB receptors. These results suggest that functional crosstalk between cannabinoid and GABA receptors regulates hippocampal synaptic plasticity.
Concentration, population, and context-dependent effects of AM251 in zebrafish.[Pubmed:26883874]
Psychopharmacology (Berl). 2016 Apr;233(8):1445-54.
RATIONALE: The function of the cannabinoid type 1 receptor (CB1-R) is poorly understood in zebrafish, and numerous inconsistent effects have been reported on it in the literature. OBJECTIVE: The objective of the present study is to determine whether differences in the reported effects of CB1-R antagonism on anxiety-like behavioural responses, dopaminergic and serotonergic responses are due to concentration, context-dependent and/or population (genotype-related) effects. METHOD: Two genetically distinct populations of zebrafish (AB and short fin (SF)) were treated with different concentrations of AM251 (0, 0.1, 1mg/L), and behavioural responses were quantified under two different contexts: one, following habituation and two, subsequently in a novel environment. The levels of dopamine, serotonin and their metabolites 3,4-dihydroxyindole acetic acid (DOPAC) and 5-hydroxyindoleacetic acid (5-HIAA) were quantified from whole-brain tissue. RESULTS: We demonstrate that a 60-min exposure to AM251 (0, 0.1, 1mg/L) does not alter behavioural performance following habituation in either populations. However, when subsequently transferred to a novel environment, zebrafish that were pre-treated with the highest dose of AM251 (1mg/L) exhibited increased anxiety-like behavioural responses including elevated absolute turn angle, freezing and bottom dwelling. We found that exposure to the highest dose of AM251 (1mg/L) for 60min increased serotonin in fish of both populations tested. In contrast, exposure to 0.1mg/L AM251 decreased, whereas to 1mg/L AM251 increased dopamine, DOPAC and 5-HIAA in fish of both populations. CONCLUSION: Our results demonstrate a genotype-independent effect of AM251 but imply that the inconsistent findings obtained after pharmacological blockade of CB1-Rs in zebrafish may be due to a combination of concentration- and environmental context-dependent effects.
The orphan receptor GPR55 is a novel cannabinoid receptor.[Pubmed:17876302]
Br J Pharmacol. 2007 Dec;152(7):1092-101.
BACKGROUND: The endocannabinoid system functions through two well characterized receptor systems, the CB1 and CB2 receptors. Work by a number of groups in recent years has provided evidence that the system is more complicated and additional receptor types should exist to explain ligand activity in a number of physiological processes. EXPERIMENTAL APPROACH: Cells transfected with the human cDNA for GPR55 were tested for their ability to bind and to mediate GTPgammaS binding by cannabinoid ligands. Using an antibody and peptide blocking approach, the nature of the G-protein coupling was determined and further demonstrated by measuring activity of downstream signalling pathways. KEY RESULTS: We demonstrate that GPR55 binds to and is activated by the cannabinoid ligand CP55940. In addition endocannabinoids including anandamide and virodhamine activate GTPgammaS binding via GPR55 with nM potencies. Ligands such as cannabidiol and abnormal cannabidiol which exhibit no CB1 or CB2 activity and are believed to function at a novel cannabinoid receptor, also showed activity at GPR55. GPR55 couples to Galpha13 and can mediate activation of rhoA, cdc42 and rac1. CONCLUSIONS: These data suggest that GPR55 is a novel cannabinoid receptor, and its ligand profile with respect to CB1 and CB2 described here will permit delineation of its physiological function(s).
Inverse agonism and neutral antagonism at cannabinoid CB1 receptors.[Pubmed:15670612]
Life Sci. 2005 Feb 4;76(12):1307-24.
There are at least two types of cannabinoid receptor, CB1 and CB2, both G protein coupled. CB1 receptors are expressed predominantly at nerve terminals and mediate inhibition of transmitter release whereas CB2 receptors are found mainly on immune cells, one of their roles being to modulate cytokine release. Endogenous cannabinoid receptor agonists also exist and these "endocannabinoids" together with their receptors constitute the "endocannabinoid system". These discoveries were followed by the development of a number of CB1- and CB2-selective antagonists that in some CB1 or CB2 receptor-containing systems also produce "inverse cannabimimetic effects", effects opposite in direction from those produced by cannabinoid receptor agonists. This review focuses on the CB1-selective antagonists, SR141716A, AM251, AM281 and LY320135, and discusses possible mechanisms by which these ligands produce their inverse effects: (1) competitive surmountable antagonism at CB1 receptors of endogenously released endocannabinoids, (2) inverse agonism resulting from negative, possibly allosteric, modulation of the constitutive activity of CB1 receptors in which CB1 receptors are shifted from a constitutively active "on" state to one or more constitutively inactive "off" states and (3) CB1 receptor-independent mechanisms, for example antagonism of endogenously released adenosine at A1 receptors. Recently developed neutral competitive CB1 receptor antagonists, which are expected to produce inverse effects through antagonism of endogenously released endocannabinoids but not by modulating CB1 receptor constitutive activity, are also discussed. So too are possible clinical consequences of the production of inverse cannabimimetic effects, there being convincing evidence that released endocannabinoids can have "autoprotective" roles.
Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists.[Pubmed:10052983]
J Med Chem. 1999 Feb 25;42(4):769-76.
As a potent, specific antagonist for the brain cannabinoid receptor (CB1), the biarylpyrazole N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2, 4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716A; 1) was the lead compound for initiating studies designed to examine the structure-activity relationships of related compounds and to search for more selective and potent cannabimimetic ligands. A series of pyrazole derivatives was designed and synthesized to aid in the characterization of the cannabinoid receptor binding sites and also to serve as potentially useful pharmacological probes. Therapeutically, such compounds may have the ability to antagonize harmful side effects of cannabinoids and cannabimimetic agents. Structural requirements for potent and selective brain cannabinoid CB1 receptor antagonistic activity included (a) a para-substituted phenyl ring at the 5-position, (b) a carboxamido group at the 3-position, and (c) a 2,4-dichlorophenyl substituent at the 1-position of the pyrazole ring. The most potent compound of this series contained a p-iodophenyl group at the 5-position, a piperidinyl carboxamide at the 3-position, and a 2,4-dichlorophenyl group at the 1-position of the pyrazole ring. The iodinated nature of this compound offers additional utility as a gamma-enriching SPECT (single photon emission computed tomography) ligand that may be useful in characterizing brain CB1 receptor binding in vivo.
Binding of the non-classical cannabinoid CP 55,940, and the diarylpyrazole AM251 to rodent brain cannabinoid receptors.[Pubmed:9335234]
Life Sci. 1997;61(14):PL 191-7.
The binding of [123I]AM251 (a radioiodinated analog of the cannabinoid CB1 receptor antagonist SR141716A) was compared to that of [3H]CP 55,940 in mouse and rat brain preparations. Scatchard analysis of the binding of [123I]AM251 and [3H]CP 55,940 to membranes prepared from mouse cerebellum, striatum and hippocampus yielded similar Bmax values (15-41 pmol/g wet wt tissue). Kd values were lower for [123I]AM251 (0.23-0.62 nM) than for [3H]CP 55,940 (1.3-4 nM). CP 55,940 and SR141716A increased dissociation of [123I]AM251 from binding sites in mouse cerebellar homogenates to a similar extent. The structurally dissimilar cannabinoid receptor ligands THC, methanandamide, WIN 55, 212-2, CP 55,940 and SR141716A were each able to fully compete with binding of both [123I]AM251 and [3H]CP 55,940 in mouse cerebellum. In vitro autoradiography demonstrated that the distribution of binding sites for [123I]AM251 in rat brain was very similar to published distributions of binding sites for [3H]CP 55,940. Together, these observations suggest that AM251 binds to the same site (the cannabinoid CB1 receptor) in rodent brains as CP 55,940. However, the binding site domains which interact with AM251 and CP 55,940 may not be identical, since IC50 values for cannabinoid receptor ligands depended on whether [123I]AM251 or [3H]CP 55,940 was used as radioligand.
123I-labeled AM251: a radioiodinated ligand which binds in vivo to mouse brain cannabinoid CB1 receptors.[Pubmed:8836622]
Eur J Pharmacol. 1996 Jul 4;307(3):331-8.
We have investigated the binding of 123I-labeled N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methy l-1 H-pyrazole-3-carboxamide (AM251), an analog of the cannabinoid receptor antagonist SR141716A [N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-me thyl-1 H-pyrazole-3-carboxamide] in the mouse brain. Following intravenous injection, the peak whole-brain uptake of about 1% of the administered activity occurred at about 2 h. By 8 h radioactivity in brain had declined to about half its peak value. High-performance liquid chromatographic analysis showed that > 70% of radioactivity extracted from brain at 2 h was still present as [123I]AM251. Co-injection of SR141716A inhibited the in vivo brain binding of [123I]AM251 dose dependently. At 2 mg/kg, the highest dose that could be tested, inhibition was 50% at 2 h post-administration. The ED50 value calculated assuming that 2 mg/kg gave near-maximal inhibition was about 0.1 mg/kg. In contrast to the brain, radioactivity in other major organs (blood, liver, kidney, heart and lung) was little affected by SR141716A. The regional binding of [123I]AM251 in the brain was consistent with the published distribution of cannabinoid receptors in rat brain, in that the order was hippocampus, striatum > cerebellum > brain stem. delta 9-Tetrahydrocannabinol co-administered intravenously at 10 mg/kg, a dose which induced catalepsy and decreased locomotor activity, decreased the 2 h brain uptake of [123I]AM251 by 10%, but this was not significant (P = 0.08). In in vitro binding assays with mouse hippocampal membranes, tetrahydrocannabinol inhibited binding of [123I]AM251 with an IC50 value of about 700 nM, compared with about 0.2 nM for SR141716A.


