Org 27569Cannabinoid CB1 receptor allosteric modulator CAS# 868273-06-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 868273-06-7 | SDF | Download SDF |
| PubChem ID | 44828492 | Appearance | Powder |
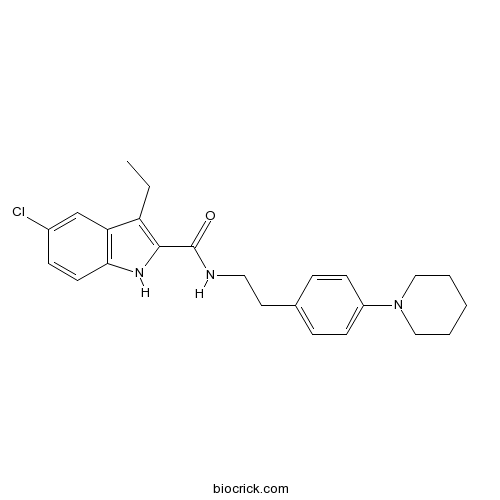
| Formula | C24H28ClN3O | M.Wt | 409.95 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 52.2 mg/mL (127.33 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 5-chloro-3-ethyl-N-[2-(4-piperidin-1-ylphenyl)ethyl]-1H-indole-2-carboxamide | ||
| SMILES | CCC1=C(NC2=C1C=C(C=C2)Cl)C(=O)NCCC3=CC=C(C=C3)N4CCCCC4 | ||
| Standard InChIKey | AHFZDNYNXFMRFQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H28ClN3O/c1-2-20-21-16-18(25)8-11-22(21)27-23(20)24(29)26-13-12-17-6-9-19(10-7-17)28-14-4-3-5-15-28/h6-11,16,27H,2-5,12-15H2,1H3,(H,26,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent CB1 receptor allosteric modulator (pEC50 = 8.24). Significantly increases binding of the CB1 agonist [3H]CP 55.940 (pKb = 5.67) and decreases binding of the CB1 inverse agonist [3H]SR 141716A (pKb = 5.95). Inhibits CB1 receptor antagonist efficacy in vitro (pKb = 7.57). |

Org 27569 Dilution Calculator

Org 27569 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4393 mL | 12.1966 mL | 24.3932 mL | 48.7864 mL | 60.983 mL |
| 5 mM | 0.4879 mL | 2.4393 mL | 4.8786 mL | 9.7573 mL | 12.1966 mL |
| 10 mM | 0.2439 mL | 1.2197 mL | 2.4393 mL | 4.8786 mL | 6.0983 mL |
| 50 mM | 0.0488 mL | 0.2439 mL | 0.4879 mL | 0.9757 mL | 1.2197 mL |
| 100 mM | 0.0244 mL | 0.122 mL | 0.2439 mL | 0.4879 mL | 0.6098 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Org 27569 is an allosteric ligand of the cannabinoid receptor CB1[1].
Org 27569 has been reported to promote the agonist CP55940 binding to purified bimane-labeled CB1 receptor and act directly on the CB1 receptor, yet at the same time inhibit receptor function. In addition, org 27569 has shown the potent inhibition of electrically evoked contractions of the mouse vas deferens by WIN55212 with the pEC50 and Emax of 8.24 ±0.12 and 45.4%, respectively. Apart from these, the pKB and Logα values for org 27569 at the putative allosteric site on the CB1 receptor are 5.67±0.23 and 1.14±0.17, respectively[1, 2].
References:
[1] Price MR1, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R, McLean A, McIntosh L, Goodwin G, Walker G, Westwood P, Marrs J, Thomson F, Cowley P, Christopoulos A, Pertwee RG, Ross RA. Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol. 2005 Nov;68(5):1484-95. Epub 2005 Aug 19.
[2] Fay JF1, Farrens DL. A key agonist-induced conformational change in the cannabinoid receptor CB1 is blocked by the allosteric ligand Org 27569. J Biol Chem. 2012 Sep 28;287(40):33873-82. Epub 2012 Jul 30.
- LY 2365109 hydrochloride
Catalog No.:BCC7677
CAS No.:868265-28-5
- Pam2CSK4
Catalog No.:BCC6247
CAS No.:868247-72-7
- SID 7969543
Catalog No.:BCC6026
CAS No.:868224-64-0
- Carasiphenol C
Catalog No.:BCN8251
CAS No.:868168-04-1
- H-Cys-OEt.HCl
Catalog No.:BCC2904
CAS No.:868-59-7
- CRF (human, rat)
Catalog No.:BCC5710
CAS No.:86784-80-7
- Astrasieversianin VII
Catalog No.:BCN2788
CAS No.:86764-11-6
- TG 100572
Catalog No.:BCC1994
CAS No.:867334-05-2
- TG 100801
Catalog No.:BCC1996
CAS No.:867331-82-6
- TG 100572 Hydrochloride
Catalog No.:BCC1995
CAS No.:867331-64-4
- TRC 051384
Catalog No.:BCC7968
CAS No.:867164-40-7
- Linsitinib
Catalog No.:BCC3697
CAS No.:867160-71-2
- Rhodiolin
Catalog No.:BCC8356
CAS No.:86831-53-0
- Rhodiosin; Herbacetin-7-O-glucorhamnoside
Catalog No.:BCN8478
CAS No.:86831-54-1
- WAY 213613
Catalog No.:BCC7442
CAS No.:868359-05-1
- 2,2,5,5-Tetramethylcyclohexane-1,4-dione
Catalog No.:BCN1324
CAS No.:86838-54-2
- Protosappanin A dimethyl acetal
Catalog No.:BCN6517
CAS No.:868405-37-2
- (+)-Puerol B 2''-O-glucoside
Catalog No.:BCN4561
CAS No.:868409-19-2
- Iristectorigenin B
Catalog No.:BCN8391
CAS No.:39012-01-6
- Carfilzomib (PR-171)
Catalog No.:BCC1145
CAS No.:868540-17-4
- 3',5'-Dimethoxybiphenyl-3-ol
Catalog No.:BCN7529
CAS No.:868666-20-0
- (R)-(-)-α-Methylhistamine dihydrobromide
Catalog No.:BCC5665
CAS No.:868698-49-1
- Umeclidinium bromide
Catalog No.:BCC2022
CAS No.:869113-09-7
- Eudesma-3,11-dien-2-one
Catalog No.:BCN7607
CAS No.:86917-79-5
Effects of the cannabinoid CB(1) receptor allosteric modulator ORG 27569 on reinstatement of cocaine- and methamphetamine-seeking behavior in rats.[Pubmed:25169627]
Drug Alcohol Depend. 2014 Oct 1;143:251-6.
BACKGROUND: Cannabinoid CB1 receptors play an essential role in drug addiction. Given the side effect profiles of orthosteric CB1 antagonism, new strategies have been attempted to modulate this target, such as CB1 receptor allosteric modulation. However, the effect of CB1 allosteric modulation in drug addiction is unknown. The present study examined the effects of the CB1 receptor allosteric modulator ORG27569 on the reinstatement of cocaine- and methamphetamine-seeking behavior in rats. METHODS: Rats were trained to self-administer 0.75 mg/kg cocaine or 0.05 mg/kg methamphetamine in 2-h daily sessions for 14 days which was followed by 7 days of extinction sessions in which rats responded on the levers with no programmed consequences. On reinstatement test sessions, rats were administered ORG27569 (1.0, 3.2, 5.6 mg/kg, i.p.) or SR141716A (3.2 mg/kg, i.p.) 10 min prior to re-exposure to cocaine- or methamphetamine-paired cues or a priming injection of cocaine (10mg/kg, i.p.) or methamphetamine (1mg/kg, i.p.). RESULTS: Both cues and a priming injection of cocaine or methamphetamine significantly reinstated the extinguished active lever responding. Pretreatment with ORG27569 resulted in a dose-related attenuation of both cue- and drug-induced reinstatement of cocaine- and methamphetamine-seeking behavior. SR141716A also exhibited similar inhibitory action on reinstatement of drug-seeking behavior. CONCLUSION: Negative allosteric modulation of CB1 receptors can produce similar functional antagonism as orthosteric CB1 receptor antagonists on reinstatement of drug-seeking behavior. Thus, ORG27569 or other negative allosteric modulators deserve further study as potentially effective pharmacotherapy for drug addiction.
In-vivo pharmacological evaluation of the CB1-receptor allosteric modulator Org-27569.[Pubmed:24603340]
Behav Pharmacol. 2014 Apr;25(2):182-5.
Several allosteric modulators (AMs) of the CB1 receptor have been characterized in vitro, including Org27569, which enhances CB1-specific binding of [H]CP55,940, but behaves as an insurmountable CB1-receptor antagonist in several biochemical assays. Although a growing body of research has investigated the molecular actions of this unusual AM, it is unknown whether these actions translate to the whole animal. The purpose of the present study was to determine whether Org27569 would produce effects in well-established mouse behavioral assays sensitive to CB1 orthosteric agonists and antagonists. Similar to the orthosteric CB1 antagonist/inverse agonist rimonabant, Org27569 reduced food intake; however, this anorectic effect occurred independently of the CB1 receptor. Org27569 did not elicit CB1-mediated effects alone and lacked efficacy in altering antinociceptive, cataleptic, and hypothermic actions of the orthosteric agonists anandamide, CP55,940, and Delta-tetrahydrocannabinol. Moreover, it did not alter the discriminative stimulus effects of anandamide in FAAH-deficient mice or Delta-tetrahydrocannabinol in wild-type mice in the drug discrimination paradigm. These findings question the utility of Org27569 as a 'gold standard' CB1 AM and underscore the need for the development of CB1 AMs with pharmacology that translates from the molecular level to the whole animal.
A key agonist-induced conformational change in the cannabinoid receptor CB1 is blocked by the allosteric ligand Org 27569.[Pubmed:22846992]
J Biol Chem. 2012 Sep 28;287(40):33873-82.
Allosteric ligands that modulate how G protein-coupled receptors respond to traditional orthosteric drugs are an exciting and rapidly expanding field of pharmacology. An allosteric ligand for the cannabinoid receptor CB1, Org 27569, exhibits an intriguing effect; it increases agonist binding, yet blocks agonist-induced CB1 signaling. Here we explored the mechanism behind this behavior, using a site-directed fluorescence labeling approach. Our results show that Org 27569 blocks conformational changes in CB1 that accompany G protein binding and/or activation, and thus inhibit formation of a fully active CB1 structure. The underlying mechanism behind this behavior is that simultaneous binding of Org 27569 produces a unique agonist-bound conformation, one that may resemble an intermediate structure formed on the pathway to full receptor activation.
Allosterism and cannabinoid CB(1) receptors: the shape of things to come.[Pubmed:18029031]
Trends Pharmacol Sci. 2007 Nov;28(11):567-72.
In 2005, the first evidence was obtained that the cannabinoid CB(1) receptor contains an allosteric binding site. The site can be recognized by synthetic small molecules, which display a markedly divergent effect on orthosteric ligand affinity versus efficacy; these small molecules are allosteric enhancers of agonist binding affinity and allosteric inhibitors of agonist signalling efficacy. Allosteric modulation heralds a new approach to the manipulation of the endocannabinoid system for therapeutic benefit; it promises to augment the existing portfolio of selective direct agonists, competitive antagonists and enzyme inhibitors.
Allosteric modulation of the cannabinoid CB1 receptor.[Pubmed:16113085]
Mol Pharmacol. 2005 Nov;68(5):1484-95.
We investigated the pharmacology of three novel compounds, Org 27569 (5-chloro-3-ethyl-1H-indole-2-carboxylic acid [2-(4-piperidin-1-yl-phenyl)-ethyl]-amide), Org 27759 (3-ethyl-5-fluoro-1H-indole-2-carboxylic acid [2-94-dimethylamino-phenyl)-ethyl]-amide), and Org 29647 (5-chloro-3-ethyl-1H-indole-2-carboxylic acid (1-benzyl-pyrrolidin-3-yl)-amide, 2-enedioic acid salt), at the cannabinoid CB1 receptor. In equilibrium binding assays, the Org compounds significantly increased the binding of the CB1 receptor agonist [3H]CP 55,940 [(1R,3R,4R)-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-4-(3-hydroxypropyl)cyclohe xan-1-ol], indicative of a positively cooperative allosteric effect. The same compounds caused a significant, but incomplete, decrease in the specific binding of the CB1 receptor inverse agonist [3H]SR 141716A [N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazol e-3-carboximide hydrochloride], indicative of a limited negative binding cooperativity. Analysis of the data according to an allosteric ternary complex model revealed that the estimated affinity of each Org compound was not significantly different when the radioligand was [3H]CP 55,940 or [3H]SR 141716A. However, the estimated cooperatively factor for the interaction between modulator and radioligand was greater than 1 when determined against [3H]CP 55,940 and less than 1 when determined against [3H]SR 141716A. [3H]CP 55,940 dissociation kinetic studies also validated the allosteric nature of the Org compounds, because they all significantly decreased radioligand dissociation. These data suggest that the Org compounds bind allosterically to the CB1 receptor and elicit a conformational change that increases agonist affinity for the orthosteric binding site. In contrast to the binding assays, however, the Org compounds behaved as insurmountable antagonists of receptor function; in the reporter gene assay, the guanosine 5'-O-(3-[35S]thio)triphosphate binding assay and the mouse vas deferens assay they elicited a significant reduction in the Emax value for CB1 receptor agonists. The data presented clearly demonstrate, for the first time, that the cannabinoid CB1 receptor contains an allosteric binding site that can be recognized by synthetic small molecule ligands.


