BML-190CB2 receptor ligand CAS# 2854-32-2 |
- CX-5461
Catalog No.:BCC3700
CAS No.:1138549-36-6
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Fludarabine Phosphate (Fludara)
Catalog No.:BCC3681
CAS No.:75607-67-9
- Bleomycin Sulfate
Catalog No.:BCC3694
CAS No.:9041-93-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 2854-32-2 | SDF | Download SDF |
| PubChem ID | 3014141 | Appearance | Powder |
| Formula | C23H23ClN2O4 | M.Wt | 426.89 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Indomethacin morpholinylamide, IMMA | ||
| Solubility | Soluble to 100 mM in DMSO with gentle warming | ||
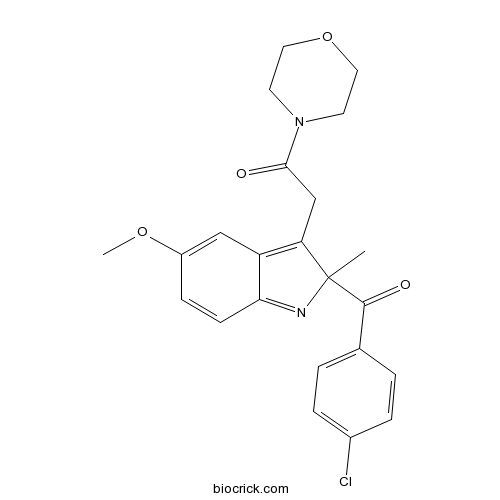
| Chemical Name | 2-[2-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl]-1-morpholin-4-ylethanone | ||
| SMILES | CC1(C(=C2C=C(C=CC2=N1)OC)CC(=O)N3CCOCC3)C(=O)C4=CC=C(C=C4)Cl | ||
| Standard InChIKey | OOKFHWWAUUITQR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H23ClN2O4/c1-23(22(28)15-3-5-16(24)6-4-15)19(14-21(27)26-9-11-30-12-10-26)18-13-17(29-2)7-8-20(18)25-23/h3-8,13H,9-12,14H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective CB2 receptor ligand (Ki values are 435 nM and > 2 μM for CB2 and CB1 respectively). |

BML-190 Dilution Calculator

BML-190 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3425 mL | 11.7126 mL | 23.4252 mL | 46.8505 mL | 58.5631 mL |
| 5 mM | 0.4685 mL | 2.3425 mL | 4.685 mL | 9.3701 mL | 11.7126 mL |
| 10 mM | 0.2343 mL | 1.1713 mL | 2.3425 mL | 4.685 mL | 5.8563 mL |
| 50 mM | 0.0469 mL | 0.2343 mL | 0.4685 mL | 0.937 mL | 1.1713 mL |
| 100 mM | 0.0234 mL | 0.1171 mL | 0.2343 mL | 0.4685 mL | 0.5856 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BML-190(IMMA) is a potent and selective CB2 receptor ligand (Ki values = 435 nM)
The cannabinoid receptor (CB1 and CB2) is a member of G-protein coupled receptor (GPCR) which plays a significant role in physiologic processes such as cognitive and immune functions.
In HEK-293 cells expressing human Cb2 receptor, BML-190 promoted the forskoline-stimulated accumulation of cAMP. BML-190 also reduced the basal level production of inositol phosphate the CB(2) receptor and 16z44-expressing cells. [1]
BML-190 played a role in LPS (lipopolysaccharide)-activated inflammation [2] and in lowering human cytokine secretion and monocytic cell neurotoxicity [3]. It activated fibroblastic colony formation [4] and moderated collagen-induce arthritis [5].
References:
[1] New DC, Wong YH. BML-190 and AM251 act as inverse agonists at the human cannabinoid CB2 receptor: signalling via cAMP and inositol phosphates. FEBS Lett. 2003 Feb 11;536(1-3):157-60. PubMed PMID: 12586356.
[2] Chang YH, Lee ST, Lin WW. Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: involvement of eicosanoids. J Cell Biochem. 2001;81(4):715-23. PubMed PMID: 11329626.
[3] Klegeris A, Bissonnette CJ, McGeer PL. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br J Pharmacol. 2003 Jun;139(4):775-86. PubMed PMID: 12813001; PubMed Central PMCID: PMC1573900.
[4] Scutt A, Williamson EM. Cannabinoids stimulate fibroblastic colony formation by bone marrow cells indirectly via CB2 receptors. Calcif Tissue Int. 2007 Jan;80(1):50-9. Epub 2007 Jan 4. PubMed PMID: 17205329.
[5] Zhang L, Zhang X, Wu P, Li H, Jin S, Zhou X, Li Y, Ye D, Chen B, Wan J. BML-111, a lipoxin receptor agonist, modulates the immune response and reduces the severity of collagen-induced arthritis. Inflamm Res. 2008 Apr;57(4):157-62. doi: 10. 1007/s00011-007-7141-z. PubMed PMID: 18648754.
[6] Zhang Q, Ma P, Cole RB, Wang G. In vitro metabolism of indomethacin morpholinylamide (BML-190), an inverse agonist for the peripheral cannabinoid receptor (CB(2)) in rat liver microsomes. Eur J Pharm Sci. 2010 Sep 11;41(1):163-72. doi: 10.1016/j.ejps.2010.06.004. Epub 2010 Jun 11. PubMed PMID: 20542112; PubMed Central PMCID: PMC2907062.
- 1-Amino-2-methylpropan-2-ol
Catalog No.:BCN1773
CAS No.:2854-16-2
- Anadoline N-oxide
Catalog No.:BCN2029
CAS No.:28513-29-3
- Malvidin-3-O-arabinoside chloride
Catalog No.:BCN3032
CAS No.:28500-04-1
- Petunidin-3-O-arabinoside chloride
Catalog No.:BCN3026
CAS No.:28500-03-0
- Petunidin-3-O-galactoside chloride
Catalog No.:BCN3024
CAS No.:28500-02-9
- Delphinidin-3-O-galactoside chloride
Catalog No.:BCN3019
CAS No.:28500-00-7
- 9-Deacetyltaxinine E
Catalog No.:BCN7227
CAS No.:284672-78-2
- 20-Deacetyltaxuspine X
Catalog No.:BCN7374
CAS No.:284672-76-0
- Tomentin
Catalog No.:BCN5180
CAS No.:28449-62-9
- Bavachalcone
Catalog No.:BCN3193
CAS No.:28448-85-3
- 4-(4-Aminophenoxy)-N-methyl-2-pyridinecarboxamide
Catalog No.:BCC8649
CAS No.:284462-37-9
- Sorafenib
Catalog No.:BCN2174
CAS No.:284461-73-0
- Theaflavin-3'-gallate
Catalog No.:BCN5421
CAS No.:28543-07-9
- 4-Benzoyloxy-2-azetidinone
Catalog No.:BCC8696
CAS No.:28562-58-5
- 7-Geranyloxy-6-methoxycoumarin
Catalog No.:BCN5181
CAS No.:28587-43-1
- Persicogenin
Catalog No.:BCN7744
CAS No.:28590-40-1
- Eicosanyl caffeate
Catalog No.:BCN7209
CAS No.:28593-90-0
- Docosyl caffeate
Catalog No.:BCN5182
CAS No.:28593-92-2
- 22-Dehydroclerosteryl acetate
Catalog No.:BCN5183
CAS No.:28594-00-5
- BIRB 796 (Doramapimod)
Catalog No.:BCC2535
CAS No.:285983-48-4
- CCG-1423
Catalog No.:BCC5581
CAS No.:285986-88-1
- Orientin
Catalog No.:BCN4984
CAS No.:28608-75-5
- Isoanhydroicaritin
Catalog No.:BCN3879
CAS No.:28610-30-2
- 8-Prenylkaempferol
Catalog No.:BCN3311
CAS No.:28610-31-3
BML-190 and AM251 act as inverse agonists at the human cannabinoid CB2 receptor: signalling via cAMP and inositol phosphates.[Pubmed:12586356]
FEBS Lett. 2003 Feb 11;536(1-3):157-60.
The aminoalkylindole BML-190 and diarylpyrazole AM251 ligands have previously been shown to bind to cannabinoid CB(2) and CB(1) receptors, respectively. In HEK-293 cells stably expressing the human CB(2) receptor, BML-190 and AM251 potentiated the forskolin-stimulated accumulation of cAMP. Moreover, the CB(2) receptor can interact productively with 16z44, a promiscuous G alpha(16/z) chimera. BML-190 and AM251 reduce the basal levels of inositol phosphate production in cells expressing the CB(2) receptor and 16z44. These results demonstrate that BML-190 and AM251 act as inverse agonists at the human CB(2) receptor acting via G alpha(i/o) and G alpha(q) family-coupled pathways.
In vitro metabolism of indomethacin morpholinylamide (BML-190), an inverse agonist for the peripheral cannabinoid receptor (CB(2)) in rat liver microsomes.[Pubmed:20542112]
Eur J Pharm Sci. 2010 Sep 11;41(1):163-72.
The in vitro metabolism of an inverse agonist of the peripheral cannabinoid receptor (CB(2)), indomethacin morpholinylamide (BML-190), has been characterized using rat liver microsomal incubation. BML-190 was found to yield at least 15 metabolic products as identified by HPLC-MS/MS analysis. Four major phase one metabolic pathways either individually, or in combination, were proposed to account for the identified metabolic products: (1) loss of the p-chlorobenzyl group, (2) hydroxylation on the indole or on the morpholine ring, (3) morpholinyl ring opening, and (4) demethylation of the methoxyl group on the indole ring.
Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation.[Pubmed:10614630]
Endocrinology. 2000 Jan;141(1):118-26.
Anandamide and 2-arachidonoylglycerol (2-AG), two endogenous ligands of the CB1 and CB2 cannabinoid receptor subtypes, inhibit the proliferation of PRL-responsive human breast cancer cells (HBCCs) through down-regulation of the long form of the PRL receptor (PRLr). Here we report that 1) anandamide and 2-AG inhibit the nerve growth factor (NGF)-induced proliferation of HBCCs through suppression of the levels of NGF Trk receptors; 2) inhibition of PRLr levels results in inhibition of the proliferation of other PRL-responsive cells, the prostate cancer DU-145 cell line; and 3) CB1-like cannabinoid receptors are expressed in HBCCs and DU-145 cells and mediate the inhibition of cell proliferation and Trk/PRLr expression. Beta-NGF-induced HBCC proliferation was potently inhibited (IC50 = 50-600 nM) by the synthetic cannabinoid HU-210, 2-AG, anandamide, and its metabolically stable analogs, but not by the anandamide congener, palmitoylethanolamide, or the selective agonist of CB2 cannabinoid receptors, BML-190. The effect of anandamide was blocked by the CB1 receptor antagonist, SR141716A, but not by the CB2 receptor antagonist, SR144528. Anandamide and HU-210 exerted a strong inhibition of the levels of NGF Trk receptors as detected by Western immunoblotting; this effect was reversed by SR141716A. When induced by exogenous PRL, the proliferation of prostate DU-145 cells was potently inhibited (IC50 = 100-300 nM) by anandamide, 2-AG, and HU-210. Anandamide also down-regulated the levels of PRLr in DU-145 cells. SR141716A attenuated these two effects of anandamide. HBCCs and DU-145 cells were shown to contain 1) transcripts for CB1 and, to a lesser extent, CB2 cannabinoid receptors, 2) specific binding sites for [3H]SR141716A that could be displaced by anandamide, and 3) a CB1 receptor-immunoreactive protein. These findings suggest that endogenous cannabinoids and CB1 receptor agonists are potential negative effectors of PRL- and NGF-induced biological responses, at least in some cancer cells.


