Tipiracil hydrochlorideThymidine phosphorylase inhibitor CAS# 183204-72-0 |
- Nemorubicin
Catalog No.:BCC4151
CAS No.:108852-90-0
- Glyoxalase I inhibitor
Catalog No.:BCC1598
CAS No.:221174-33-0
- Doxifluridine
Catalog No.:BCC4903
CAS No.:3094-09-5
- Plumbagin
Catalog No.:BCN2586
CAS No.:481-42-5
- Fluorouracil (Adrucil)
Catalog No.:BCC2135
CAS No.:51-21-8
- Deguelin
Catalog No.:BCN4804
CAS No.:522-17-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 183204-72-0 | SDF | Download SDF |
| PubChem ID | 9903778 | Appearance | Powder |
| Formula | C9H12Cl2N4O2 | M.Wt | 279.12 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 50 mg/mL (179.13 mM; Need ultrasonic) DMSO : < 1 mg/mL (insoluble or slightly soluble) DMF : < 1 mg/mL (insoluble) | ||
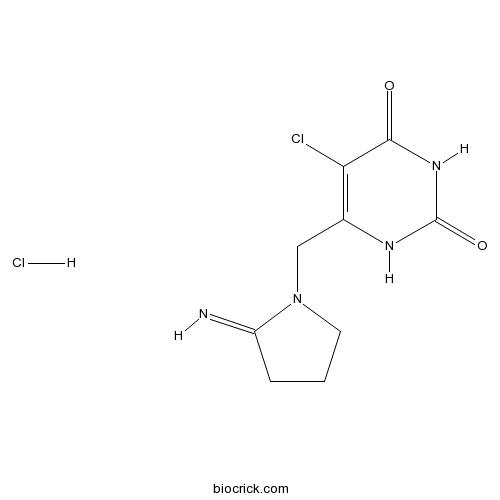
| Chemical Name | 5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1H-pyrimidine-2,4-dione;hydrochloride | ||
| SMILES | C1CC(=N)N(C1)CC2=C(C(=O)NC(=O)N2)Cl.Cl | ||
| Standard InChIKey | KGHYQYACJRXCAT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H11ClN4O2.ClH/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11;/h11H,1-4H2,(H2,12,13,15,16);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tipiracil hydrochloride is an inhibitor of thymidine phosphorylase. | |||||
| Targets | thymidine phosphorylase | |||||

Tipiracil hydrochloride Dilution Calculator

Tipiracil hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5827 mL | 17.9134 mL | 35.8269 mL | 71.6538 mL | 89.5672 mL |
| 5 mM | 0.7165 mL | 3.5827 mL | 7.1654 mL | 14.3308 mL | 17.9134 mL |
| 10 mM | 0.3583 mL | 1.7913 mL | 3.5827 mL | 7.1654 mL | 8.9567 mL |
| 50 mM | 0.0717 mL | 0.3583 mL | 0.7165 mL | 1.4331 mL | 1.7913 mL |
| 100 mM | 0.0358 mL | 0.1791 mL | 0.3583 mL | 0.7165 mL | 0.8957 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tipiracil is an inhibitor of Thymidine phosphorylase (TP).
Thymidine phosphorylase (TP)is a key enzyme in the pyrimidine nucleoside salvage pathway. It catalyses the reversible phosphorylation of thymidine, and thereby generate thymine and 2-deoxy-D-ribose-1-phosphate.
Tipiracil (TPI) and trifluridine (FTD) are active components of TAS-102 at a molecular ratio of 1:0.5, which is a novel oral nucleoside antitumor agent in clinical trials. Oral administered TPI and FTD co-treatment may differ from that of i.v. administration of FTD alone. [1] When FTD is administered orally, it is rapidly degraded to its inactive form in the intestines and the liver (first-pass effect) [2], but the combination with TPI helps to maintain adequate FTD plasma concentrations [3]. TPI thus, potentiates the antitumor activity of FTD, and the optimal molecular ratio of FTD to TPI has been proven to be 1:0.5. [3]
References:
1. Tsukihara H1, Nakagawa F2, Sakamoto K et al. Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102, together with bevacizumab, cetuximab, or panitumumab on human colorectal cancer xenografts. Oncol Rep. 2015 May;33(5):2135-42.
2. Dexter DL, Wolberg WH, Ansfield FJ, Helson L and Heidelberger C: The clinical pharmacology of 5-trifluoro-methyl-2'-deoxyuridine. Cancer Res 32: 247-253, 1972.
3. Fukushima M, Suzuki N, Emura T et al. Structure and activity of specific inhibitors of thymidine phosphorylase to potentiate the function of antitumor 2'-deoxyribonucleosides. biochem Pharmacol 59: 1227-1236, 2000.
- Guanylin (human)
Catalog No.:BCC7204
CAS No.:183200-12-6
- Cabazitaxel
Catalog No.:BCC4966
CAS No.:183133-96-2
- Fabiatrin
Catalog No.:BCN2920
CAS No.:18309-73-4
- 3,4-O-Isopropylidene shikimic acid
Catalog No.:BCN1147
CAS No.:183075-03-8
- Didrovaltrate
Catalog No.:BCN7124
CAS No.:18296-45-2
- Valepotriate
Catalog No.:BCN2351
CAS No.:18296-44-1
- Dibritannilactone B
Catalog No.:BCN7776
CAS No.:1829580-18-8
- (9Z,12Z)-N-Benzyloctadeca-9,12-dienamide
Catalog No.:BCN1518
CAS No.:18286-71-0
- Thalidezine
Catalog No.:BCN7763
CAS No.:18251-36-0
- SB 225002
Catalog No.:BCC8077
CAS No.:182498-32-4
- TPMPA
Catalog No.:BCC6903
CAS No.:182485-36-5
- Lomitapide
Catalog No.:BCC5570
CAS No.:182431-12-5
- 1,7-Dihydroxy-2,3-methylenedioxyxanthone
Catalog No.:BCN7543
CAS No.:183210-63-1
- AM251
Catalog No.:BCC4412
CAS No.:183232-66-8
- 2-Acetoxy-3-deacetoxycaesaldekarin E
Catalog No.:BCN7476
CAS No.:18326-06-2
- 5-(1-Piperazinyl)benzofuran-2-carboxamide
Catalog No.:BCC8717
CAS No.:183288-46-2
- Erlotinib Hydrochloride
Catalog No.:BCC3645
CAS No.:183319-69-9
- OSI-420
Catalog No.:BCC4472
CAS No.:183320-51-6
- Erlotinib
Catalog No.:BCC1557
CAS No.:183321-74-6
- CPPG
Catalog No.:BCC6872
CAS No.:183364-82-1
- Apicidin
Catalog No.:BCC3599
CAS No.:183506-66-3
- Cleroindicin A
Catalog No.:BCC8916
CAS No.:176598-06-4
- CYN 154806
Catalog No.:BCC5823
CAS No.:183658-72-2
- Penthiopyrad
Catalog No.:BCC8072
CAS No.:183675-82-3
[Antitumor Molecular Mechanism of Trifluridine and Tipiracil Hydrochloride (TAS-102: TFTD)].[Pubmed:26809521]
Gan To Kagaku Ryoho. 2016 Jan;43(1):8-14.
Treatment options for patients with metastatic colorectal cancer (mCRC), who are refractory to standard chemotherapy, are limited. In a global multicenter randomized double-blind phase III study (RECOURSE study), TAS-102 (TFTD) administration significantly improved overall survival rate with favorable safety profile in mCRC patients refractory to standard chemotherapy (HR=0.68, p<0.001). TFTD was approved initially in Japan in March 2014 and is currently under review by health authorities in the United States and Europe. TFTD is expected to play an important role in salvage-line treatment for patients with mCRC. In this review, we present the history of its clinical development and the experimental data that elucidate the underlying molecular mechanism of action of TFTD and its key component, trifluridine.
[Initial Evaluation of the Efficacy and Safety of Tablets Containing Trifluridine and Tipiracil Hydrochloride--Safety Measures Devised by a Multidisciplinary Team Including a Pharmaceutical Outpatient Clinic].[Pubmed:26197742]
Gan To Kagaku Ryoho. 2015 Jul;42(7):817-20.
In May 2014, tablets containing both trifluridine and Tipiracil hydrochloride (Lonsurf(R) tablets) were launched in Japan ahead of other countries, for the treatment of advanced/relapsed unresectable colorectal cancer. The benefits of these tablets in terms of a new therapeutic option have been demonstrated. However, the manufacturer has requested healthcare professionals to help develop safety measures for the appropriate and safe use of the tablets. In this study, we evaluated the efficacy and safety of the tablets in 16 patients who received the tablets at our hospital. Among the 4 evaluable patients, none achieved a complete or partial response. One patient (25.0%) had stable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) Guidelines outlined in the General Rules of the Study of Colorectal Cancer (The 8th Edition). Lonsurf(R) is considered to be a third-line (or later) treatment. Among the 16 cases studied, Lonsurf(R) was used as a third-, fourth-, and fifth-line treatment in 9, 6, and 1 cases, respectively. Therefore, Grade 3 or worse toxicities were a potential concern. Despite a high incidence of Grade 3 or worse neutropenia (7 of the 16 patients [43.8%]), none of the patients were hospitalized due to neutropenia or other treatment-related adverse events. Pharmacists have made 126 proposals to physicians regarding the use of Lonsurf(R), 121 (96.0%) of which have been adopted. All of the adverse reactions experienced by our patients were resolved after supportive therapy.
Self-Reported Adherence to Trifluridine and Tipiracil Hydrochloride for Metastatic Colorectal Cancer: A Retrospective Cohort Study.[Pubmed:27513940]
Oncology. 2016;91(4):224-230.
BACKGROUND: A novel oral agent that consists of trifluridine and Tipiracil hydrochloride (TFTD) has been established as salvage-line treatment for metastatic colorectal cancer (mCRC). Adherence to TFTD is crucial to maintaining appropriate curative effects. This study sought to clarify adherence to TFTD and identify candidate factors deteriorating adherence at our institution. METHODS: A total of 50 consecutive mCRC patients who received TFTD monotherapy between June 1, 2014 and July 31, 2015 were analyzed in this study. Adherence to TFTD was checked by pharmacists using a self-reported treatment diary and interviewing nonadherents at a pharmaceutical outpatient clinic. The adherence rate was defined as the number of patient intakes per 20 scheduled intakes in one cycle. We retrospectively surveyed the factors from the electronic patient record associated with reduced adherence. We measured relative dose intensity, defined as the dose intensity divided by the initial dose (each in milligrams per square meter per week). RESULTS: Patient characteristics were as follows: males/females, 20/30; median age, 61 years (range, 34-83 years); performance status 0/1, 37/13. Median relative dose intensity of TFTD was 91.0%. Adherence rates were 95.0% for the first cycle of TFTD, 97.3% for the second cycle, 98.0% for the third cycle, and 98.2% for the fourth cycle. Factors associated with deteriorated adherence to TFTD were nausea/vomiting/decreased appetite (27.1%, 23 instances), pain (25.9%, 22 instances), neutropenia (11.8%, 10 instances), and missed dose (4.7%, 4 instances). Increased nonadherence to TFTD was associated with Eastern Cooperative Oncology Group performance status 1, while increased TFTD adherence in the first cycle was associated with prior regimens >/=4. CONCLUSIONS: The high frequency of treatment-related gastrointestinal disorder is the main factor affecting adherence to TFTD. Intensive supportive care in the management of these symptoms could assist adequate adherence to TFTD in mCRC patients.


