HydroxyisoleucineCAS# 55399-93-4 |
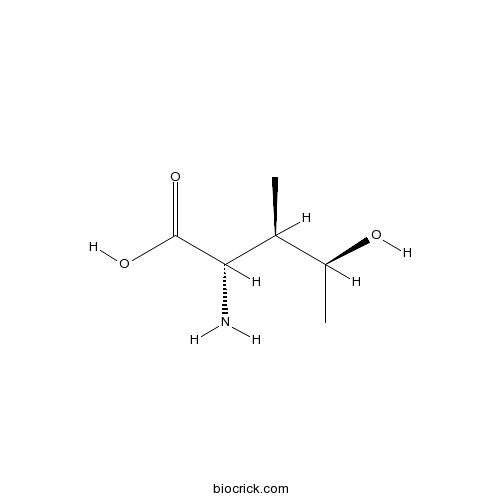
- 4-Hydroxyisoleucine
Catalog No.:BCN1211
CAS No.:781658-23-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 55399-93-4 | SDF | Download SDF |
| PubChem ID | 6918732 | Appearance | White crystalline powder |
| Formula | C6H13NO3 | M.Wt | 147.17 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (2S,3R,4S)-4-Hydroxyisoleucine;(4S)-4-Hydroxy-L-isoleucine | ||
| Solubility | H2O : 125 mg/mL (849.36 mM; Need ultrasonic) | ||
| Chemical Name | (2S,3R,4S)-2-amino-4-hydroxy-3-methylpentanoic acid | ||
| SMILES | CC(C(C)O)C(C(=O)O)N | ||
| Standard InChIKey | OSCCDBFHNMXNME-YUPRTTJUSA-N | ||
| Standard InChI | InChI=1S/C6H13NO3/c1-3(4(2)8)5(7)6(9)10/h3-5,8H,7H2,1-2H3,(H,9,10)/t3-,4-,5-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Hydroxyisoleucine Dilution Calculator

Hydroxyisoleucine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.7949 mL | 33.9743 mL | 67.9486 mL | 135.8973 mL | 169.8716 mL |
| 5 mM | 1.359 mL | 6.7949 mL | 13.5897 mL | 27.1795 mL | 33.9743 mL |
| 10 mM | 0.6795 mL | 3.3974 mL | 6.7949 mL | 13.5897 mL | 16.9872 mL |
| 50 mM | 0.1359 mL | 0.6795 mL | 1.359 mL | 2.7179 mL | 3.3974 mL |
| 100 mM | 0.0679 mL | 0.3397 mL | 0.6795 mL | 1.359 mL | 1.6987 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Baohuoside II
Catalog No.:BCN2888
CAS No.:55395-07-8
- Vidarabine
Catalog No.:BCC4877
CAS No.:5536-17-4
- Beclomethasone dipropionate
Catalog No.:BCC4257
CAS No.:5534-09-8
- Petasiphenone
Catalog No.:BCC8100
CAS No.:162616-81-1
- Soyasaponin II
Catalog No.:BCN1418
CAS No.:55319-36-3
- Atherosperminine
Catalog No.:BCN8208
CAS No.:5531-98-6
- Costunolide
Catalog No.:BCN5740
CAS No.:553-21-9
- Xanthyletin
Catalog No.:BCN6722
CAS No.:553-19-5
- Thonzonium Bromide
Catalog No.:BCC5636
CAS No.:553-08-2
- Tiamulin
Catalog No.:BCC9179
CAS No.:55297-95-5
- (Z)-Falcarindiol
Catalog No.:BCN8495
CAS No.:55297-87-5
- Atractylodin
Catalog No.:BCN6292
CAS No.:55290-63-6
- Lithium carbonate
Catalog No.:BCC7970
CAS No.:554-13-2
- Methazolamide
Catalog No.:BCC2318
CAS No.:554-57-4
- Deferasirox Fe3+ chelate
Catalog No.:BCC1521
CAS No.:554435-83-5
- 8beta-(4-Hydroxytigloyloxy)ovatifolin
Catalog No.:BCN7122
CAS No.:554449-27-3
- Boc- D-1-Nal-OH
Catalog No.:BCC3283
CAS No.:55447-00-2
- Anisodamine hydrobromide
Catalog No.:BCC8119
CAS No.:55449-49-5
- Z-Asp(OtBu)-OH.H2O
Catalog No.:BCC2789
CAS No.:5545-52-8
- Chimonanthine
Catalog No.:BCN7824
CAS No.:5545-89-1
- Jujuboside A
Catalog No.:BCN4949
CAS No.:55466-04-1
- Jujuboside B
Catalog No.:BCN4950
CAS No.:55466-05-2
- Indole-3-glyoxylamide
Catalog No.:BCN6802
CAS No.:5548-10-7
- Isosaxalin
Catalog No.:BCN5741
CAS No.:55481-86-2
Engineering a short-chain dehydrogenase/reductase for the stereoselective production of (2S,3R,4S)-4-hydroxyisoleucine with three asymmetric centers.[Pubmed:29057974]
Sci Rep. 2017 Oct 20;7(1):13703.
Fenugreek is a dietary supplement for anti-aging and human health. (2S,3R,4S)-4-Hydroxyisoleucine (4-HIL), which is extracted from fenugreek seeds, is expected to be a promising orally active drug for diabetes and diabetic nephropathy because of its insulinotropic effect. Although several chemical synthesis methods of 4-HIL have been proposed, these methods require multistep reactions to control the stereochemistry of 4-HIL. In this study, we modified the key enzyme 4-HIL dehydrogenase (HILDH) to overcome the biggest limitation in commercial-scale production of 4-HIL. As a result, an effective one-step carbonyl reduction to produce (2S,3R,4S)-4-HIL was successfully accomplished with strict stereoselectivity (>99% de). Mass production of (2S,3R,4S)-4-HIL by our synthetic method could have a significant contribution to the prevention of diabetes, dyslipidemia, and Alzheimer's disease. (120 words/200 words).
Preclinical Toxicological Evaluation of IDM01: The Botanical Composition of 4-Hydroxyisoleucine- and Trigonelline-based Standardized Fenugreek Seed Extract.[Pubmed:28539737]
Pharmacognosy Res. 2017 Apr-Jun;9(2):138-150.
OBJECTIVE: To evaluate acute oral toxicity (AOT), subchronic (90-day repeated dose) toxicity, mutagenicity, and genotoxicity potential of IDM01, the botanical composition of 4-Hydroxyisoleucine- and trigonelline-based standardized fenugreek (Trigonella foenum-graecum L) seed extract in laboratory rats. MATERIALS AND METHODS: The AOT and subchronic (90-day repeated dose) toxicity were evaluated using Sprague-Dawley rats as per the Organisation for Economic Co-operation and Development (OECD) guidelines No. 423 and No. 408, respectively. During the subchronic study, the effects on body weight, food and water consumption, organ weights with hematology, clinical biochemistry, and histology were studied. The mutagenicity and genotoxicity of IDM01 were evaluated by reverse mutation assay (Ames test, OECD guideline No. 471) and chromosome aberration test (OECD guideline No. 473), respectively. RESULTS: The IDM01 did not show mortality or treatment-related adverse signs during acute (limit dose of 2000 mg/kg) and subchronic (90-day repeated dose of 250, 500, and 1000 mg/kg with 28 days of recovery period) administration. The IDM01 showed oral median lethal dose (LD50) >2000 mg/kg during AOT study. The no-observed adverse effect level (NOAEL) of IDM01 was 500 mg/kg. IDM01 did not show mutagenicity up to a concentration of 5000 mug/plate during Ames test and did not induce structural chromosomal aberrations up to 50 mg/culture. CONCLUSIONS: IDM01 was found safe during preclinical acute and subchronic (90-day repeated dose) toxicity in rats without mutagenicity or genotoxicity. SUMMARY: Acute oral toxicity, subchronic (90-day) oral toxicity, mutagenicity and genotoxicity of IDM01 (4-Hydroxyisoleucine- and trigonelline-based standardized fenugreek seed extract) was evaluated.The median lethal dose, LD50, of IDM01 was more than 2000 mg/kg of body weight in rats.No observed adverse effect level (NOAEL) of IDM01 was 500 mg/kg of body weight in rats.IDM01 was found safe during acute and subchronic oral toxicity studies in rats without mutagenicity or genotoxicity potetial. Abbreviations Used: 2-AA: 2-aminoanthracene; 2-AF: 2-aminofluorene; 4 NQNO: 4-nitroquinolene-N-oxide; 4HI: 4-Hydroxyisoleucine; ANOVA: Analysis of variance; AOT: Acute oral toxicity; DM: Diabetes mellitus; IDM01: The Botanical composition of 4-Hydroxyisoleucine- and trigonelline-based standardized fenugreek seed extract; LD50: Median lethal dose; MMS: Methyl methanesulfonate; NAD: No abnormality detected; OECD: Organisation for Economic Co-operation and Development; SD: Standard deviation; UV: Ultraviolet; VC: Vehicle control. 2-AA: 2-aminoanthracene; 2-AF: 2-aminofluorene; 4 NQNO: 4-nitroquinolene-N-oxide; 4HI: 4-Hydroxyisoleucine; ANOVA: Analysis of variance; AOT: Acute oral toxicity; DM: Diabetes mellitus; IDM01: The Botanical composition of 4-Hydroxyisoleucine- and trigonelline-based standardized fenugreek seed extract; LD50: Median lethal dose; MMS: Methyl methanesulfonate; NAD: No abnormality detected; OECD: Organisation for Economic Co-operation and Development; SD: Standard deviation; UV: Ultraviolet; VC: Vehicle control.
Molecular Docking Analysis of Phytic Acid and 4-hydroxyisoleucine as Cyclooxygenase-2, Microsomal Prostaglandin E Synthase-2, Tyrosinase, Human Neutrophil Elastase, Matrix Metalloproteinase-2 and -9, Xanthine Oxidase, Squalene Synthase, Nitric Oxide Synthase, Human Aldose Reductase, and Lipoxygenase Inhibitors.[Pubmed:29142407]
Pharmacogn Mag. 2017 Oct;13(Suppl 3):S512-S518.
Background: The phytoconstituents phytic acid and 4-Hydroxyisoleucine have been reported to posses various biological properties. Objective: This prompted us to carry out the docking study on these two ligands (phytic acid & 4-Hydroxyisoleucine) against eleven targeted enzymes. Materials and Methods: Phytic acid & 4-Hydroxyisoleucine were evaluated on the docking behaviour of cyclooxygenase-2 (COX-2), microsomal prostaglandin E synthase-2 (mPGES-2), tyrosinase, human neutrophil elastase (HNE), matrix metalloproteinase (MMP 2 and 9), xanthine oxidase (XO), squalene synthase (SQS), nitric oxide synthase (NOS), human aldose reductase (HAR) and lipoxygenase (LOX) using Discovery Studio Version 3.1 (except for LOX, where Autodock 4.2 tool was used). Results: Docking and binding free energy analysis revealed that phytic acid exhibited the maximum binding energy for four target enzymes such as COX-2, mPGES-2, tyrosinase and HNE. Interestingly, we found that 4-Hydroxyisoleucine has the potential to dock and bind with all of the eleven targeted enzymes. Conclusion: This present study has paved a new insight in understanding 4-Hydroxyisoleucine as potential inhibitor against COX-2, mPGES-2, tyrosinase, HNE, MMP 2, MMP 9, XO, SQS, NOS, HAR and LOX. SUMMARY: 4-Hydroxyisoleucine has the potential to dock and bind with all 11targeted enzymes such as (cyclooxygenase-2 [COX-2], microsomal prostaglandin E synthase-2 [mPGES-2], tyrosinase, human neutrophil elastase [HNE], matrix metalloproteinase [MMP-2 and -9], xanthine oxidase, squalene synthase, nitric oxide synthase, human aldose reductase, and lipoxygenase)Moreover, docking studies and binding free energy calculations revealed that phytic acid exhibited the maximum binding energy for four target enzymes such as COX-2, mPGES-2, tyrosinase, and HNE; however, for other six target enzymes, it fails to dock. Abbreviations used: COX-2: Cyclooxygenase-2, mPGES-2: Microsomal prostaglandin E synthase-2, HNE: Human neutrophil elastase, MMP-2 and -9: Matrix metalloproteinase-2 and -9, XO: Xanthine oxidase, SQS: Squalene synthase, NOS: Nitric oxide synthase, HAR: Human aldose reductase, LOX: Lipoxygenase, ADME: Absorption, distribution, metabolism, and excretion, TOPKAT: Toxicity Prediction by Computer-assisted Technology.
Lipid lowering agents of natural origin: An account of some promising chemotypes.[Pubmed:28987600]
Eur J Med Chem. 2017 Nov 10;140:331-348.
The role of natural products in the drug development and discovery has been phenomenal. There has been an enormous interest in exploring all possible natural sources to identify structures exhibiting pronounced hypolipidemic activity albeit with no toxicity. The present review describes the profile of some interesting naturally occurring compounds and their derivatives as potential hypolipidemic agents. Some of the interesting natural chemotypes that can control the increased levels of plasma lipids and discussed in this review are compactin, lovastatin, gugglesterone, berberine, lupeol, phytol, polyprenol, aegeline, 4-Hydroxyisoleucine, alpha-asarone, resveratrol, esculeoside A, swertiamarin, rutin, saucerneol B, curcumin and a clerodane diterpene.
Attempt to simultaneously generate three chiral centers in 4-hydroxyisoleucine with microbial carbonyl reductases.[Pubmed:28698052]
Bioorg Med Chem. 2018 Apr 1;26(7):1327-1332.
A panel of microorganisms was screened for selective reduction ability towards a racemic mixture of prochiral 2-amino-3-methyl-4-ketopentanoate (rac-AMKP). Several of the microorganisms tested produced greater than 0.5mM 4-Hydroxyisoleucine (HIL) from rac-AMKP, and the stereoselectivity of HIL formation was found to depend on the taxonomic category to which the microorganism belonged. The enzymes responsible for the AMKP-reducing activity, ApAR and FsAR, were identified from two of these microorganisms, Aureobasidium pullulans NBRC 4466 and Fusarium solani TG-2, respectively. Three AMKP reducing enzymes, ApAR, FsAR, and the previously reported BtHILDH, were reacted with rac-AMKP, and each enzyme selectively produced a specific composition of HIL stereoisomers. The enzymes appeared to have different characteristics in recognition of the stereostructure of the substrate AMKP and in control of the 4-hydroxyl group configuration in the HIL product.
4-Hydroxyisoleucine from Fenugreek (Trigonella foenum-graecum): Effects on Insulin Resistance Associated with Obesity.[Pubmed:27879673]
Molecules. 2016 Nov 22;21(11). pii: molecules21111596.
Obesity and insulin resistance (IR) are interdependent multifactorial processes that cannot be understood separately. Obesity leads to systemic inflammation and increased levels of free fatty acids that provoke IR and lipotoxicity. At the same time, IR exacerbates adipose cell dysfunction, resulting in chronic inflammation and major lipotoxic effects on nonadipose tissues. 4-Hydroxyisoleucine (4-OHIle), a peculiar nonprotein amino acid isolated from fenugreek (Trigonella foenum-graecum) seeds, exhibits interesting effects on IR related to obesity. 4-OHIle increases glucose-induced insulin release, and the insulin response mediated by 4-OHIle depends on glucose concentration. The beneficial effects observed are related to the regulation of blood glucose, plasma triglycerides, total cholesterol, free fatty acid levels, and the improvement of liver function. The mechanism of action is related to increased Akt phosphorylation and reduced activation of Jun N-terminal kinase (JNK)1/2, extracellular signal-regulated kinase (ERK)1/2, p38 mitogen-activated protein kinase (MAPK), and nuclear factor (NF)-kappaB. Here, we present a review of the research regarding the insulinotropic and insulin-sensitising activity of 4-OHIle in in vitro and in vivo models.
A strategy for L-isoleucine dioxygenase screening and 4-hydroxyisoleucine production by resting cells.[Pubmed:28430004]
Bioengineered. 2018 Jan 1;9(1):72-79.
L-Isoleucine dioxygenase (IDO) specifically converts L-isoleucine(L-Ile) to 4-Hydroxyisoleucine(4-HIL). To obtain IDO with improved activity, a strategy was developed that is dependent on the restoration of succinate-minus E. coli cell growth by the coupling of L-Ile hydroxylation and the oxidation of alpha-ketoglutarate(alpha-KGA) to succinate. Five mutants were obtained with this strategy, and the characteristics of IDO(M3), which exhibited the highest activity, were studied. The catalytic efficiency, thermal stability and catalytic rate of IDO(M3) were significantly improved compared with those of wild-type IDO. Moreover, an efficient method for the biotransformation of 4-HIL by resting cells expressing IDO(M3) was developed, with which 151.9 mmol of 4-HIL/L (22.4 g/L) was synthesized in 12 h while the substrates seldom exhibited additional consumption.


