4-HydroxyisoleucineCAS# 781658-23-9 |
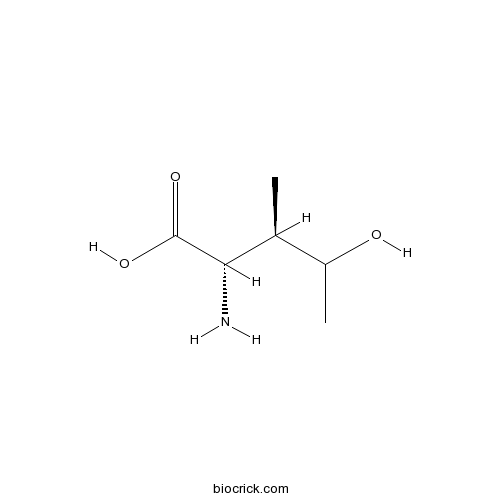
- Hydroxyisoleucine
Catalog No.:BCN8402
CAS No.:55399-93-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 781658-23-9 | SDF | Download SDF |
| PubChem ID | 2773624 | Appearance | White powder |
| Formula | C6H13NO3 | M.Wt | 147.17 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | L-4-Hydroxyisoleucine;6001-78-8 | ||
| Solubility | Soluble in water | ||
| Chemical Name | (2S,3R)-2-amino-4-hydroxy-3-methylpentanoic acid | ||
| SMILES | CC(C(C)O)C(C(=O)O)N | ||
| Standard InChIKey | OSCCDBFHNMXNME-DSDZBIDZSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 4-Hydroxyisoleucine has antidepressant-like, antidyslipidemic, and antihyperglycemic effects. It acts to improve insulin resistance by promoting mitochondrial biogenesis in high fructose diet fed STZ induced diabetic rats, it also has beneficial effects on low-grade inflammation.L-4-Hydroxyisoleucine is a natural product from Trigonella foenum-graecum L. |
| Targets | TNF-α | AMPK | Akt | GSK-3 | ROS | NF-kB | JNK | ERK | p38MAPK | 5-HT Receptor |
| In vitro | 4-Hydroxyisoleucine ameliorates fatty acid-induced insulin resistance and inflammatory response in skeletal muscle cells.[Pubmed: 25109277]Mol Cell Endocrinol. 2014 Sep;395(1-2):51-60.The 4-Hydroxyisoleucine (4-HIL), an unusual amino acid isolated from the seeds of Trigonella foenum-graecum was investigated for its metabolic effects to ameliorate free fatty acid-induced insulin resistance in skeletal muscle cells.
|
| In vivo | 4-Hydroxyisoleucine improves insulin resistance by promoting mitochondrial biogenesis and act through AMPK and Akt dependent pathway.[Pubmed: 25454462]Fitoterapia. 2014 Dec;99:307-17.4-Hydroxyisoleucine (4-HIL) is an unusual amino acid isolated from fenugreek seeds (Trigonella foenum graecum L). Various studies have shown that it acts as an antidiabetic agent yet its mechanism of action is not clear.
4-hydroxyisoleucine an unusual amino acid as antidyslipidemic and antihyperglycemic agent.[Pubmed: 16246556 ]Bioorg Med Chem Lett. 2006 Jan 15;16(2):293-6.Trigonella foenum-graecum, commonly known as fenugreek, is an annual herbaceous plant.
|
| Cell Research | In vitro anti-hyperglycemic activity of 4-hydroxyisoleucine derivatives.[Pubmed: 25636873]Phytomedicine. 2015 Jan 15;22(1):66-70.The nonproteinogenic amino acid, 4-Hydroxyisoleucine (1) has been isolated in large quantities from the fenugreek (T. foenum-graecum) seeds.
|
| Animal Research | Antidepressant-like effect of 4-hydroxyisoleucine from Trigonella foenum graecum L. seeds in mice.[Reference: WebLink]Biomed. Aging Pathol., 2012, 2(3):121-5.Trigonella foenum graecum L. (Fabaceae), also known as fenugreek, is one of the oldest medicinal plants, and has a long history of medical use in traditional and modern literature. 4-Hydroxyisoleucine (4-HI) constitutes about is 80% of the total content of free amino acids in Trigonella foenum graecum seeds.
|
| Structure Identification | Microbiologyopen. 2013 Jun;2(3):471-81.A novel l-isoleucine-4'-dioxygenase and l-isoleucine dihydroxylation cascade in Pantoea ananatis.[Pubmed: 23554367 ]A unique operon structure has been identified in the genomes of several plant- and insect-associated bacteria. The distinguishing feature of this operon is the presence of tandem hilA and hilB genes encoding dioxygenases belonging to the PF13640 and PF10014 (BsmA) Pfam families, respectively.
|

4-Hydroxyisoleucine Dilution Calculator

4-Hydroxyisoleucine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.7949 mL | 33.9743 mL | 67.9486 mL | 135.8973 mL | 169.8716 mL |
| 5 mM | 1.359 mL | 6.7949 mL | 13.5897 mL | 27.1795 mL | 33.9743 mL |
| 10 mM | 0.6795 mL | 3.3974 mL | 6.7949 mL | 13.5897 mL | 16.9872 mL |
| 50 mM | 0.1359 mL | 0.6795 mL | 1.359 mL | 2.7179 mL | 3.3974 mL |
| 100 mM | 0.0679 mL | 0.3397 mL | 0.6795 mL | 1.359 mL | 1.6987 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- CDPPB
Catalog No.:BCC7610
CAS No.:781652-57-1
- MK-0974
Catalog No.:BCC1756
CAS No.:781649-09-0
- DAMGO
Catalog No.:BCC6958
CAS No.:78123-71-4
- Okadaic acid
Catalog No.:BCC2464
CAS No.:78111-17-8
- Aztreonam
Catalog No.:BCC2557
CAS No.:78110-38-0
- 2-Acetylfluorene
Catalog No.:BCC8516
CAS No.:781-73-7
- (R)-(+)-8-Hydroxy-DPAT hydrobromide
Catalog No.:BCC6929
CAS No.:78095-19-9
- Fmoc-Lys(Fmoc)-OH
Catalog No.:BCC3521
CAS No.:78081-87-5
- MMPX
Catalog No.:BCC6692
CAS No.:78033-08-6
- 1,3-Diacetylvilasinin
Catalog No.:BCN4580
CAS No.:78012-28-9
- Zeylasterone
Catalog No.:BCN8057
CAS No.:78012-25-6
- Linalool
Catalog No.:BCN6339
CAS No.:78-70-6
- YM155
Catalog No.:BCC2251
CAS No.:781661-94-7
- Nirtetralin
Catalog No.:BCN3755
CAS No.:78185-63-4
- 20(S)-Ginsenoside Rh2
Catalog No.:BCN1070
CAS No.:78214-33-2
- Paroxetine HCl
Catalog No.:BCC5054
CAS No.:78246-49-8
- [Orn5]-URP
Catalog No.:BCC5985
CAS No.:782485-03-4
- Hydroxysafflor yellow A
Catalog No.:BCN1049
CAS No.:78281-02-4
- Nepafenac
Catalog No.:BCC1258
CAS No.:78281-72-8
- Ecliptasaponin A
Catalog No.:BCN3843
CAS No.:78285-90-2
- 7-Ethylcamptothecin
Catalog No.:BCN2480
CAS No.:78287-27-1
- H-D-1-Nal-OH
Catalog No.:BCC3281
CAS No.:78306-92-0
- MRK 016
Catalog No.:BCC6070
CAS No.:783331-24-8
- MLN120B
Catalog No.:BCC1772
CAS No.:783348-36-7
4-Hydroxyisoleucine attenuates the inflammation-mediated insulin resistance by the activation of AMPK and suppression of SOCS-3 coimmunoprecipitation with both the IR-beta subunit as well as IRS-1.[Pubmed:26887316]
Mol Cell Biochem. 2016 Mar;414(1-2):95-104.
It is known that 4-Hydroxyisoleucine (4-HIL) from seeds of Trigonella foenum-graecum has beneficial effects on low-grade inflammation; therefore, the insulin signaling as well as the anti-inflammatory effects of 4-HIL in TNF-alpha-induced insulin resistance in C2C12 myotubes was studied with an aim to dissect out the mechanism(s) of the inflammation-mediated insulin resistance. TNF-alpha suppressed insulin-stimulated glucose transport rate and increased Ser-307 phosphorylation of insulin receptor substrate-1 (IRS-1). However, the treatment of 4-Hydroxyisoleucine enhanced insulin-stimulated glucose transport rate via the activation of AMP-activated protein kinase (AMPK) in a dose-dependent manner. 4-HIL also increases the tyrosine phosphorylation of both IR-beta and IRS-1. Moreover, coimmunoprecipitation (Co-IP) of insulin receptor-beta (IR-beta) subunit with IRS-1 was found to be increased by 4-Hydroxyisoleucine. Concentration of SOCS-3 protein and coimmunoprecipitation of SOCS-3 protein with both the IR-beta subunit as well as IRS-1 was found to be decreased by 4-HIL. We conclude that the 4-Hydroxyisoleucine reverses the insulin resistance by the activation of AMPK and suppression of SOCS-3 coimmunoprecipitation with both the IR-beta subunit as well as IRS-1.
4-Hydroxyisoleucine ameliorates fatty acid-induced insulin resistance and inflammatory response in skeletal muscle cells.[Pubmed:25109277]
Mol Cell Endocrinol. 2014 Sep;395(1-2):51-60.
The 4-Hydroxyisoleucine (4-HIL), an unusual amino acid isolated from the seeds of Trigonella foenum-graecum was investigated for its metabolic effects to ameliorate free fatty acid-induced insulin resistance in skeletal muscle cells. An incubation of L6 myotubes with palmitate inhibited insulin stimulated-glucose uptake and -translocation of glucose transporter 4 (GLUT4) to the cell surface. Addition of 4-HIL strongly prevented this inhibition. We then examined the insulin signaling pathway, where 4-HIL effectively inhibited the ability of palmitate to reduce insulin-stimulated phosphorylation of insulin receptor substrate-1 (IRS-1), protein kinase B (PKB/AKT), AKT substrate of 160 kD (AS160) and glycogen synthase kinase 3beta (GSK-3beta) in L6 myotubes. Moreover, 4-HIL presented strong inhibition on palmitate-induced production of reactive oxygen species (ROS) and associated inflammation, as the activation of NF-kappaB, JNK1/2, ERK1/2 and p38 MAPK was greatly reduced. 4-HIL also inhibited inflammation-stimulated IRS-1 serine phosphorylation and restored insulin-stimulated IRS-1 tyrosine phosphorylation in the presence of palmitate, leading to enhanced insulin sensitivity. These findings suggested that 4-HIL could inhibit palmitate-induced, ROS-associated inflammation and restored insulin sensitivity through regulating IRS-1 function.
A novel l-isoleucine-4'-dioxygenase and l-isoleucine dihydroxylation cascade in Pantoea ananatis.[Pubmed:23554367]
Microbiologyopen. 2013 Jun;2(3):471-81.
A unique operon structure has been identified in the genomes of several plant- and insect-associated bacteria. The distinguishing feature of this operon is the presence of tandem hilA and hilB genes encoding dioxygenases belonging to the PF13640 and PF10014 (BsmA) Pfam families, respectively. The genes encoding HilA and HilB from Pantoea ananatis AJ13355 were cloned and expressed in Escherichia coli. The culturing of E. coli cells expressing hilA (E. coli-HilA) or both hilA and hilB (E. coli-HilAB) in the presence of l-isoleucine resulted in the conversion of l-isoleucine into two novel biogenic compounds: l-4'-isoleucine and l-4,4'-dihydroxyisoleucine, respectively. In parallel, two novel enzymatic activities were detected in the crude cell lysates of the E. coli-HilA and E. coli-HilAB strains: l-isoleucine, 2-oxoglutarate: oxygen oxidoreductase (4'-hydroxylating) (HilA) and l-4'-hydroxyisoleucine, 2-oxoglutarate: oxygen oxidoreductase (4-hydroxylating) (HilB), respectively. Two hypotheses regarding the physiological significance of C-4(4')-hydroxylation of l-isoleucine in bacteria are also discussed. According to first hypothesis, the l-isoleucine dihydroxylation cascade is involved in synthesis of dipeptide antibiotic in P. ananatis. Another unifying hypothesis is that the C-4(4')-hydroxylation of l-isoleucine in bacteria could result in the synthesis of signal molecules belonging to two classes: 2(5H)-furanones and analogs of N-acyl homoserine lactone.
In vitro anti-hyperglycemic activity of 4-hydroxyisoleucine derivatives.[Pubmed:25636873]
Phytomedicine. 2015 Jan 15;22(1):66-70.
The nonproteinogenic amino acid, 4-Hydroxyisoleucine (1) has been isolated in large quantities from the fenugreek (T. foenum-graecum) seeds. Few novel derivatives (3-11 and 13-18) were prepared from the naturally occurring 4-Hydroxyisoleucine (1) and screened for their in vitro glucose uptake stimulatory effect in L-6 skeletal muscle cells. The derivatives 6, 7, 8, 10 and 11 exhibited better glucose uptake stimulatory activity than parent compound, 4-Hydroxyisoleucine at 5 and 10microM concentrations and compounds 7 and 11 enhanced translocation of insulin sensitive glucose transporters-4 in skeletal muscle cells.
4-hydroxyisoleucine an unusual amino acid as antidyslipidemic and antihyperglycemic agent.[Pubmed:16246556]
Bioorg Med Chem Lett. 2006 Jan 15;16(2):293-6.
Trigonella foenum-graecum, commonly known as fenugreek, is an annual herbaceous plant. From the seeds of T. foenum-graecum an unusual amino acid, 4-Hydroxyisoleucine 5, has been isolated, which significantly decreased the plasma triglyceride levels by 33% (P<0.002), total cholesterol (TC) by 22% (P<0.02), and free fatty acids by 14%, accompanied by an increase in HDL-C/TC ratio by 39% in the dyslipidemic hamster model.
4-Hydroxyisoleucine improves insulin resistance by promoting mitochondrial biogenesis and act through AMPK and Akt dependent pathway.[Pubmed:25454462]
Fitoterapia. 2014 Dec;99:307-17.
4-Hydroxyisoleucine (4-HIL) is an unusual amino acid isolated from fenugreek seeds (Trigonella foenum graecum L). Various studies have shown that it acts as an antidiabetic agent yet its mechanism of action is not clear. We therefore investigated the effect 4-HIL on the high fructose diet fed streptozotocin induced diabetic rats and L6 myotubes. 4-HIL (50 mg/kg) has improved blood lipid profile, glucose tolerance and insulin sensitivity in a diabetic rat model. It has increased the glucose uptake in L6 myotubes in AMPK-dependent manner and upregulated the expression of genes (PGC-1alpha, PGC-1beta, CPT 1 and CPT 2), which have role in mitochondrial biogenesis and energy metabolism in the liver, skeletal muscles as well as in L6 myotubes. Interestingly, it also increased the AMPK and Akt expression along with their phosphorylated forms in the liver and muscle tissues of treated animals. Altogether we concluded that 4-HIL acts to improve insulin resistance by promoting mitochondrial biogenesis in high fructose diet fed STZ induced diabetic rats.


