Lithium carbonateMood stabilizer; inhibits Na+/K+ ATPase pump activity CAS# 554-13-2 |
- Ferrostatin-1 (Fer-1)
Catalog No.:BCC2323
CAS No.:347174-05-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 554-13-2 | SDF | Download SDF |
| PubChem ID | 11125 | Appearance | Powder |
| Formula | Li2CO3 | M.Wt | 73.89 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | dilithium;carbonate | ||
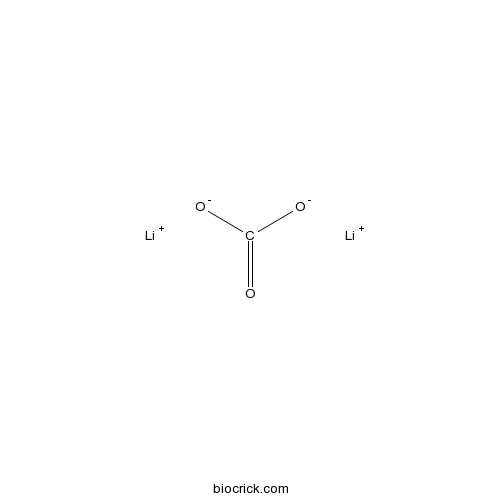
| SMILES | [Li+].[Li+].C(=O)([O-])[O-] | ||
| Standard InChIKey | XGZVUEUWXADBQD-UHFFFAOYSA-L | ||
| Standard InChI | InChI=1S/CH2O3.2Li/c2-1(3)4;;/h(H2,2,3,4);;/q;2*+1/p-2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Mood stabilizer; thought to act by reducing catecholamine neurotransmitter concentration, through an effect on the Na+/K+ ATPase pump. Effective in the treatment of bipolar disorder. Inhibits GSK-3 in vivo. |

Lithium carbonate Dilution Calculator

Lithium carbonate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 13.5336 mL | 67.6682 mL | 135.3363 mL | 270.6726 mL | 338.3408 mL |
| 5 mM | 2.7067 mL | 13.5336 mL | 27.0673 mL | 54.1345 mL | 67.6682 mL |
| 10 mM | 1.3534 mL | 6.7668 mL | 13.5336 mL | 27.0673 mL | 33.8341 mL |
| 50 mM | 0.2707 mL | 1.3534 mL | 2.7067 mL | 5.4135 mL | 6.7668 mL |
| 100 mM | 0.1353 mL | 0.6767 mL | 1.3534 mL | 2.7067 mL | 3.3834 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hydroxyisoleucine
Catalog No.:BCN8402
CAS No.:55399-93-4
- Baohuoside II
Catalog No.:BCN2888
CAS No.:55395-07-8
- Vidarabine
Catalog No.:BCC4877
CAS No.:5536-17-4
- Beclomethasone dipropionate
Catalog No.:BCC4257
CAS No.:5534-09-8
- Petasiphenone
Catalog No.:BCC8100
CAS No.:162616-81-1
- Soyasaponin II
Catalog No.:BCN1418
CAS No.:55319-36-3
- Atherosperminine
Catalog No.:BCN8208
CAS No.:5531-98-6
- Costunolide
Catalog No.:BCN5740
CAS No.:553-21-9
- Xanthyletin
Catalog No.:BCN6722
CAS No.:553-19-5
- Thonzonium Bromide
Catalog No.:BCC5636
CAS No.:553-08-2
- Tiamulin
Catalog No.:BCC9179
CAS No.:55297-95-5
- (Z)-Falcarindiol
Catalog No.:BCN8495
CAS No.:55297-87-5
- Methazolamide
Catalog No.:BCC2318
CAS No.:554-57-4
- Deferasirox Fe3+ chelate
Catalog No.:BCC1521
CAS No.:554435-83-5
- 8beta-(4-Hydroxytigloyloxy)ovatifolin
Catalog No.:BCN7122
CAS No.:554449-27-3
- Boc- D-1-Nal-OH
Catalog No.:BCC3283
CAS No.:55447-00-2
- Anisodamine hydrobromide
Catalog No.:BCC8119
CAS No.:55449-49-5
- Z-Asp(OtBu)-OH.H2O
Catalog No.:BCC2789
CAS No.:5545-52-8
- Chimonanthine
Catalog No.:BCN7824
CAS No.:5545-89-1
- Jujuboside A
Catalog No.:BCN4949
CAS No.:55466-04-1
- Jujuboside B
Catalog No.:BCN4950
CAS No.:55466-05-2
- Indole-3-glyoxylamide
Catalog No.:BCN6802
CAS No.:5548-10-7
- Isosaxalin
Catalog No.:BCN5741
CAS No.:55481-86-2
- Mollugin
Catalog No.:BCN5742
CAS No.:55481-88-4
Lithium carbonate and coenzyme Q10 reduce cell death in a cell model of Machado-Joseph disease.[Pubmed:27878228]
Braz J Med Biol Res. 2016 Nov 21;49(12):e5805.
Machado-Joseph disease (MJD) or spinocerebellar ataxia type 3 (SCA3) is an autosomal dominant neurodegenerative disorder caused by expansion of the polyglutamine domain of the ataxin-3 (ATX3) protein. MJD/SCA3 is the most frequent autosomal dominant ataxia in many countries. The mechanism underlying MJD/SCA3 is thought to be mainly related to protein misfolding and aggregation leading to neuronal dysfunction followed by cell death. Currently, there are no effective treatments for patients with MJD/SCA3. Here, we report on the potential use of Lithium carbonate and coenzyme Q10 to reduce cell death caused by the expanded ATX3 in cell culture. Cell viability and apoptosis were evaluated by MTT assay and by flow cytometry after staining with annexin V-FITC/propidium iodide. Treatment with Lithium carbonate and coenzyme Q10 led to a significant increase in viability of cells expressing expanded ATX3 (Q84). In addition, we found that the increase in cell viability resulted from a significant reduction in the proportion of apoptotic cells. Furthermore, there was a significant change in the expanded ATX3 monomer/aggregate ratio after Lithium carbonate and coenzyme Q10 treatment, with an increase in the monomer fraction and decrease in aggregates. The safety and tolerance of both drugs are well established; thus, our results indicate that Lithium carbonate and coenzyme Q10 are good candidates for further in vivo therapeutic trials.
Optimization of Microporous Carbon Structures for Lithium-Sulfur Battery Applications in Carbonate-Based Electrolyte.[Pubmed:28060452]
Small. 2017 Mar;13(11).
Developing appropriate sulfur cathode materials in carbonate-based electrolyte is an important research subject for lithium-sulfur batteries. Although several microporous carbon materials as host for sulfur reveal the effect, methods for producing microporous carbon are neither easy nor well controllable. Moreover, due to the complexity and limitation of microporous carbon in their fabrication process, there has been rare investigation of influence on electrochemical behavior in the carbonate-based electrolyte for lithium-sulfur batteries by tuning different micropore size(0-2 nm) of carbon host. Here, we demonstrate an immediate carbonization process, self-activation strategy, which can produce microporous carbon for a sulfur host from alkali-complexes. Besides, by changing different alkali-ion in the previous complex, the obtained microporous carbon exhibits a major portion of ultramicropore (<0.7 nm, from 54.9% to 25.8%) and it is demonstrated that the micropore structure of the host material plays a vital role in confining sulfur molecule. When evaluated as cathode materials in a carbonate-based electrolyte for Li-S batteries, such microporous carbon/sulfur composite can provide high reversible capacity, cycling stability and good rate capability.
In Situ Generation of Poly (Vinylene Carbonate) Based Solid Electrolyte with Interfacial Stability for LiCoO2 Lithium Batteries.[Pubmed:28251055]
Adv Sci (Weinh). 2016 Nov 10;4(2):1600377.
Nowadays it is extremely urgent to seek high performance solid polymer electrolyte that possesses both interfacial stability toward lithium/graphitic anodes and high voltage cathodes for high energy density solid state batteries. Inspired by the positive interfacial effect of vinylene carbonate additive on solid electrolyte interface, a novel poly (vinylene carbonate) based solid polymer electrolyte is presented via a facile in situ polymerization process in this paper. It is manifested that poly (vinylene carbonate) based solid polymer electrolyte possess a superior electrochemical stability window up to 4.5 V versus Li/Li(+) and considerable ionic conductivity of 9.82 x 10(-5) S cm(-1) at 50 degrees C. Moreover, it is demonstrated that high voltage LiCoO2/Li batteries using this solid polymer electrolyte display stable charge/discharge profiles, considerable rate capability, excellent cycling performance, and decent safety characteristic. It is believed that poly (vinylene carbonate) based electrolyte can be a very promising solid polymer electrolyte candidate for high energy density lithium batteries.
Lithium Carbonate Recovery from Cathode Scrap of Spent Lithium-Ion Battery: A Closed-Loop Process.[Pubmed:28081362]
Environ Sci Technol. 2017 Feb 7;51(3):1662-1669.
A closed-loop process to recover Lithium carbonate from cathode scrap of lithium-ion battery (LIB) is developed. Lithium could be selectively leached into solution using formic acid while aluminum remained as the metallic form, and most of the other metals from the cathode scrap could be precipitated out. This phenomenon clearly demonstrates that formic acid can be used for lithium recovery from cathode scrap, as both leaching and separation reagent. By investigating the effects of different parameters including temperature, formic acid concentration, H2O2 amount, and solid to liquid ratio, the leaching rate of Li can reach 99.93% with minor Al loss into the solution. Subsequently, the leaching kinetics was evaluated and the controlling step as well as the apparent activation energy could be determined. After further separation of the remaining Ni, Co, and Mn from the leachate, Li2CO3 with the purity of 99.90% could be obtained. The final solution after Lithium carbonate extraction can be further processed for sodium formate preparation, and Ni, Co, and Mn precipitates are ready for precursor preparation for cathode materials. As a result, the global recovery rates of Al, Li, Ni, Co, and Mn in this process were found to be 95.46%, 98.22%, 99.96%, 99.96%, and 99.95% respectively, achieving effective resources recycling from cathode scrap of spent LIB.
In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3.[Pubmed:12942141]
Neuropsychopharmacology. 2004 Jan;29(1):32-8.
There is considerable interest in the possibility that small-molecule glycogen synthase kinase-3 inhibitors may have utility in the treatment of bipolar disorder, since glycogen synthase kinase-3 is a target of lithium. Although the in vitro inhibition of glycogen synthase kinase-3 by lithium occurs with a K(i) of 1-2 mM, the degree of inhibition of this enzyme in the mammalian brain at therapeutically relevant concentrations has not fully been established. The transcription factor beta-catenin is an established marker of glycogen synthase kinase-3 inactivation because cytoplasmic levels are increased by inhibition of the enzyme. In this study, we measured beta-catenin protein levels after treatment with therapeutically relevant doses of lithium, valproate, and carbamazepine. Western blot revealed that 9 days of treatment with lithium and valproate, but not carbamazepine, increased beta-catenin protein levels in soluble fractions from the frontal cortex. The level of beta-catenin in the particulate fraction, which is not directly regulated by glycogen synthase kinase-3, did not change with any of the three drugs. Furthermore, real-time PCR revealed that lithium significantly decreased beta-catenin mRNA levels, which may represent compensation for an increase in beta-catenin stability. These results strongly suggest that lithium significantly inhibits brain glycogen synthase kinase-3 in vivo at concentrations relevant for the treatment of bipolar disorder.
Overview of the mechanism of action of lithium in the brain: fifty-year update.[Pubmed:10826655]
J Clin Psychiatry. 2000;61 Suppl 9:5-15.
Since its discovery, lithium has been shown to act upon various neurotransmitter systems at multiple levels of signaling in the brain. Lithium, affecting each neurotransmitter system within complex interactive neuronal networks, is suggested to restore the balance among aberrant signaling pathways in critical regions of the brain. Recent molecular studies have revealed the action of lithium on signal transduction mechanisms, such as phosphoinositide hydrolysis, adenylyl cyclase, G protein, glycogen synthase kinase-3beta, protein kinase C, and its substrate myristoylated alanine-rich C kinase substrate. Such effects are thought to trigger long-term changes in neuronal signaling patterns that account for the prophylactic properties of lithium in the treatment of bipolar disorder. Through its effects on glycogen synthase kinase-3beta and protein kinase C, lithium may alter the level of phosphorylation of cytoskeletal proteins, which leads to neuroplastic changes associated with mood stabilization. Chronic lithium regulates transcriptional factors, which in turn may modulate the expression of a variety of genes that compensate for aberrant signaling associated with the pathophysiology of bipolar disorder. Future studies on long-term neuroplastic changes caused by lithium in the brain will set the stage for new drug-discovery opportunities.


