3-Epidehydropachymic acidCAS# 168293-15-0 |
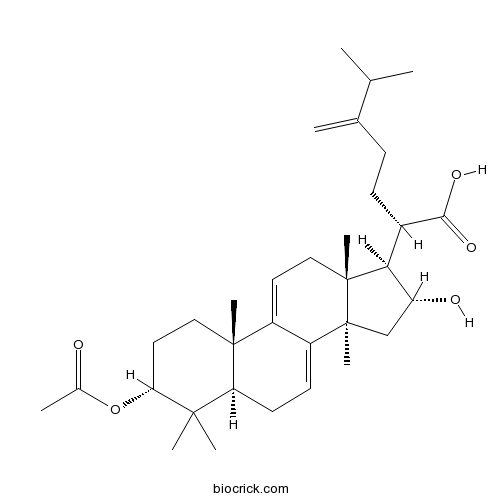
- Dehydropachymic acid
Catalog No.:BCN3648
CAS No.:77012-31-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 168293-15-0 | SDF | Download SDF |
| PubChem ID | 15226716 | Appearance | Powder |
| Formula | C33H50O5 | M.Wt | 526.8 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R)-2-[(3R,5R,10S,13R,14R,16R,17R)-3-acetyloxy-16-hydroxy-4,4,10,13,14-pentamethyl-2,3,5,6,12,15,16,17-octahydro-1H-cyclopenta[a]phenanthren-17-yl]-6-methyl-5-methylideneheptanoic acid | ||
| SMILES | CC(C)C(=C)CCC(C1C(CC2(C1(CC=C3C2=CCC4C3(CCC(C4(C)C)OC(=O)C)C)C)C)O)C(=O)O | ||
| Standard InChIKey | RWIALJIVPUCERT-ROLXBSRMSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Separation Science and Technology, 2014, 49(17):2765-2771.Acid-Alkali Extraction ofTriterpene Acids from Poria and Preparative Separation by High-Speed Counter-Current Chromatography.[Reference: WebLink]
|

3-Epidehydropachymic acid Dilution Calculator

3-Epidehydropachymic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8983 mL | 9.4913 mL | 18.9825 mL | 37.9651 mL | 47.4563 mL |
| 5 mM | 0.3797 mL | 1.8983 mL | 3.7965 mL | 7.593 mL | 9.4913 mL |
| 10 mM | 0.1898 mL | 0.9491 mL | 1.8983 mL | 3.7965 mL | 4.7456 mL |
| 50 mM | 0.038 mL | 0.1898 mL | 0.3797 mL | 0.7593 mL | 0.9491 mL |
| 100 mM | 0.019 mL | 0.0949 mL | 0.1898 mL | 0.3797 mL | 0.4746 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-O-Acetyl-16 alpha-hydroxydehydrotrametenolic acid
Catalog No.:BCN1531
CAS No.:168293-14-9
- 3-O-Acetyl-16 alpha-hydroxytrametenolic acid
Catalog No.:BCN1532
CAS No.:168293-13-8
- C-Veratroylglycol
Catalog No.:BCN1102
CAS No.:168293-10-5
- 3,5-Dimethoxy-3'-hydroxybibenzyl
Catalog No.:BCN8112
CAS No.:168281-05-8
- Rimonabant
Catalog No.:BCC4414
CAS No.:168273-06-1
- Evofolin B
Catalog No.:BCN1101
CAS No.:168254-96-4
- Wilforol C
Catalog No.:BCN1100
CAS No.:168254-95-3
- Nortenuazonic acid
Catalog No.:BCN1847
CAS No.:16820-44-3
- Agitoxin 2
Catalog No.:BCC8026
CAS No.:168147-41-9
- Taxin B
Catalog No.:BCN6945
CAS No.:168109-52-2
- Mearnsetin
Catalog No.:BCN6560
CAS No.:16805-10-0
- cis-Miyabenol C
Catalog No.:BCN3347
CAS No.:168037-22-7
- Asiaticoside
Catalog No.:BCN1011
CAS No.:16830-15-2
- Oxymatrine
Catalog No.:BCN1103
CAS No.:16837-52-8
- Tacrine hydrochloride
Catalog No.:BCC6869
CAS No.:1684-40-8
- Compound 401
Catalog No.:BCC7622
CAS No.:168425-64-7
- Rhodiocyanoside A
Catalog No.:BCN7852
CAS No.:168433-86-1
- Epifriedelanol
Catalog No.:BCN1104
CAS No.:16844-71-6
- SHU 9119
Catalog No.:BCC6019
CAS No.:168482-23-3
- Z-D-Val-OH
Catalog No.:BCC2732
CAS No.:1685-33-2
- Dehydrogeijerin
Catalog No.:BCN7531
CAS No.:16850-91-2
- Fosbretabulin (Combretastatin A4 Phosphate (CA4P)) Disodium
Catalog No.:BCC4600
CAS No.:168555-66-6
- H-Asp(OMe)-OH.HCl
Catalog No.:BCC2889
CAS No.:16856-13-6
- AIDA
Catalog No.:BCC6841
CAS No.:168560-79-0
Effects of conjugated linoleic acid/n-3 and resistance training on muscle quality and expression of atrophy-related ubiquitin ligases in middle-aged mice with high-fat dietinduced obesity.[Pubmed:29036761]
J Exerc Nutrition Biochem. 2017 Sep 30;21(3):11-18.
PURPOSE: To investigate the effects of conjugated linoleic acid (CLA)/n-3 supplements and resistance exercise training (RT) for 20 weeks on muscle quality and genes related to protein synthesis/degradation in middle-aged mice with high-fat diet (HFD)-induced obesity. METHODS: Nine-month-old C57BL/6 male mice were randomly assigned to five groups: 1) normal diet (C), 2) high-fat diet (H), 3) H+RT (HRT), 4) H+CLA/n-3 (H-CN), and 5) H+RT+CLA/n-3 (H-RTCN). HFD groups were given a diet containing 60% fat for 20 weeks, and exercised groups underwent progressive RT using weighted ladder climbing. The CLA/n-3 mixed diet contained 1% CLA and 1% n-3. Grip strength was assessed, and triceps were removed. RT-PCR was used to analyze transcript levels. RESULTS: Grip strength of the H group was significantly lower than that of the C group; however, those in the H-CN, H-RT, and H-RTN groups were significantly greater than that in the H group. However, the muscle quality was significantly greater only in the H-RT group compared with the H and H-CN groups. Akt expression decreased in the H-CN, H-RT, and H-RTCN groups compared with those in the C and H groups, whereas mammalian target of rapamycin expression increased in the H, H-CN, H-RT, and H-RTCN groups compared with that in the C group. However, atrogin1 was significantly downregulated in the H-RTCN group compared with that in the H and H-CN groups, and MuRF1 expression was also decreased in the H-RT and H-RTCN groups. Interestingly, atrogin1 and MuRF1 were downregulated in the H-RTCN group compared with that in the H-CN group. CONCLUSION: HFD-mediated gene expression involved in protein degradation was attenuated following 20-week RT with CLA/n-3. Furthermore, RT with or without CLA/n-3 improved grip strength and muscle quality in middle-aged mice during HFD. Therefore, RT with CLA/n-3 during HFD may improve muscle strength and quality by suppressing protein degradation.
Facile Synthesis and Characterization of L-Aspartic Acid Coated Iron Oxide Magnetic Nanoparticles (IONPs) For Biomedical Applications.[Pubmed:29036735]
Drug Res (Stuttg). 2018 May;68(5):280-285.
Natural L-aspartic acid coated iron oxide magnetic nanoparticles (Asp@IONPs) were prepared by a one pot, in-situ and green co-precipitation method in an aqueous medium. Functionalized iron oxide magnetic nanoparticles (IONPs) were characterized by Vibrating Sample Magnetometer (VSM), X-ray diffraction (XRD), differential scanning calorimetry (DSC), Fourier transform infrared spectroscopy (FTIR), Scanning electron microscopy (SEM) and Transmission electron microscopy (TEM) techniques. Cellular toxicity of IONPs was also investigated on HEK-293 cell lines. The results showed that the zeta potential of Asp@IONPs was about -21.1 mV and the average size was 17.80+/-3.09 nm. Cell toxicity results show that as prepared IONPs are biocompatible. Asp@IONPs show the possibility of using these nanoparticles in the development of in vitro and in vivo biomedical fields due to do not possess a toxic effect, good zeta-potential and related small and narrow size distribution.
High Interspecimen Variability in Nucleic Acid Extraction Efficiency Necessitates the Use of Spike-In Control for Accurate qPCR-based Measurement of Plasma Cell-Free DNA Levels.[Pubmed:29036313]
Lab Med. 2017 Nov 8;48(4):332-338.
Objective: To assess the interspecimen variability associated with plasma DNA extraction in order to provide insight regarding the necessity to use an exogenous spike-in control when measuring cell-free DNA (cfDNA) levels using quantitative polymerase chain reaction (qPCR). Methods: Plasma specimens were obtained from 8 healthy individuals, 20 patients with cardiovascular disease risk factors, and 54 patients diagnosed with acute stroke. Specimens were spiked with an exogenous oligonucleotide fragment, and total DNA was extracted via automated solid phase anion exchange. We determined recovery of the exogenous fragment via qPCR and used this information to calculate DNA extraction efficiency. Results: Plasma DNA extraction efficiencies varied dramatically between specimens, ranging from 22.9% to 88.1%, with a coefficient of variance of 28.9%. No significant differences in DNA extraction efficiencies were observed between patient populations. Conclusions: We strongly recommend the use of an exogenous spike-in control to account for variance in plasma DNA extraction efficiency when assessing cell free DNA (cfDNA) levels by qPCR in future biomarker investigations.
2'-Fluoroarabinonucleic acid modification traps G-quadruplex and i-motif structures in human telomeric DNA.[Pubmed:29036537]
Nucleic Acids Res. 2017 Nov 16;45(20):11535-11546.
Human telomeres and promoter regions of genes fulfill a significant role in cellular aging and cancer. These regions comprise of guanine and cytosine-rich repeats, which under certain conditions can fold into G-quadruplex (G4) and i-motif structures, respectively. Herein, we use UV, circular dichroism and NMR spectroscopy to study several human telomeric sequences and demonstrate that G4/i-motif-duplex interconversion kinetics are slowed down dramatically by 2'-beta-fluorination and the presence of G4/i-motif-duplex junctions. NMR-monitored kinetic experiments on 1:1 mixtures of native and modified C- and G-rich human telomeric sequences reveal that strand hybridization kinetics are controlled by G4 or i-motif unfolding. Furthermore, we provide NMR evidence for the formation of a hybrid complex containing G4 and i-motif structures proximal to a duplex DNA segment at neutral pH. While the presence of i-motif and G4 folds may be mutually exclusive in promoter genome sequences, our results suggest that they may co-exist transiently as intermediates in telomeric sequences.
Metabolomics and Biochemical Approaches Link Salicylic Acid Biosynthesis to Cyanogenesis in Peach Plants.[Pubmed:29036663]
Plant Cell Physiol. 2017 Dec 1;58(12):2057-2066.
Despite the long-established importance of salicylic acid (SA) in plant stress responses and other biological processes, its biosynthetic pathways have not been fully characterized. The proposed synthesis of SA originates from chorismate by two distinct pathways: the isochorismate and phenylalanine (Phe) ammonia-lyase (PAL) pathways. Cyanogenesis is the process related to the release of hydrogen cyanide from endogenous cyanogenic glycosides (CNglcs), and it has been linked to plant plasticity improvement. To date, however, no relationship has been suggested between the two pathways. In this work, by metabolomics and biochemical approaches (including the use of [13C]-labeled compounds), we provide strong evidences showing that CNglcs turnover is involved, at least in part, in SA biosynthesis in peach plants under control and stress conditions. The main CNglcs in peach are prunasin and amygdalin, with mandelonitrile (MD), synthesized from phenylalanine, controlling their turnover. In peach plants MD is the intermediary molecule of the suggested new SA biosynthetic pathway and CNglcs turnover, regulating the biosynthesis of both amygdalin and SA. MD-treated peach plants displayed increased SA levels via benzoic acid (one of the SA precursors within the PAL pathway). MD also provided partial protection against Plum pox virus infection in peach seedlings. Thus, we propose a third pathway, an alternative to the PAL pathway, for SA synthesis in peach plants.


