Evofolin BCAS# 168254-96-4 |
-
(-)-Evofolin B
Catalog No.:BCN9159
CAS No.:1961305-60-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 168254-96-4 | SDF | Download SDF |
| PubChem ID | 5317306 | Appearance | Powder |
| Formula | C17H18O6 | M.Wt | 318.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | 1961305-60-1 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
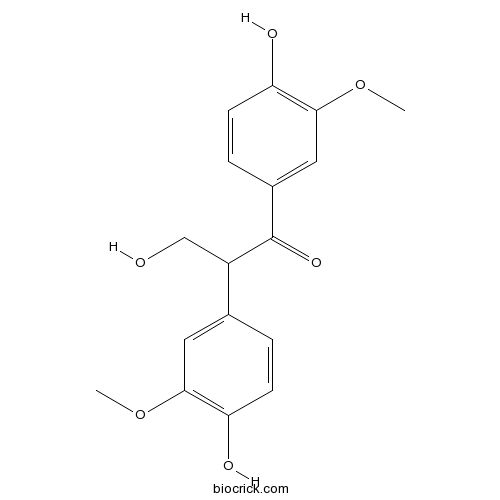
| Chemical Name | 3-hydroxy-1,2-bis(4-hydroxy-3-methoxyphenyl)propan-1-one | ||
| SMILES | COC1=C(C=CC(=C1)C(CO)C(=O)C2=CC(=C(C=C2)O)OC)O | ||
| Standard InChIKey | QMYGRGKKZBRZKH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H18O6/c1-22-15-7-10(3-5-13(15)19)12(9-18)17(21)11-4-6-14(20)16(8-11)23-2/h3-8,12,18-20H,9H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Evofolin B exhibits quinone reductase (QR) inducing activity, with observed CD (concentration required to double induction) values of 16.4 microM. |

Evofolin B Dilution Calculator

Evofolin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1417 mL | 15.7085 mL | 31.4169 mL | 62.8338 mL | 78.5423 mL |
| 5 mM | 0.6283 mL | 3.1417 mL | 6.2834 mL | 12.5668 mL | 15.7085 mL |
| 10 mM | 0.3142 mL | 1.5708 mL | 3.1417 mL | 6.2834 mL | 7.8542 mL |
| 50 mM | 0.0628 mL | 0.3142 mL | 0.6283 mL | 1.2567 mL | 1.5708 mL |
| 100 mM | 0.0314 mL | 0.1571 mL | 0.3142 mL | 0.6283 mL | 0.7854 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Wilforol C
Catalog No.:BCN1100
CAS No.:168254-95-3
- Nortenuazonic acid
Catalog No.:BCN1847
CAS No.:16820-44-3
- Agitoxin 2
Catalog No.:BCC8026
CAS No.:168147-41-9
- Taxin B
Catalog No.:BCN6945
CAS No.:168109-52-2
- Mearnsetin
Catalog No.:BCN6560
CAS No.:16805-10-0
- cis-Miyabenol C
Catalog No.:BCN3347
CAS No.:168037-22-7
- Boc-D-Threoninol(Bzl)
Catalog No.:BCC2703
CAS No.:168034-31-9
- NXY-059
Catalog No.:BCC4955
CAS No.:168021-79-2
- Triptonine B
Catalog No.:BCN3095
CAS No.:168009-85-6
- Otophylloside B 4'''-O-beta-D-oleandropyranoside
Catalog No.:BCN7512
CAS No.:168001-54-5
- Stigmasta-4,22-diene-3beta,6beta-diol
Catalog No.:BCN1533
CAS No.:167958-89-6
- Taxachitriene B
Catalog No.:BCN6951
CAS No.:167906-75-4
- Rimonabant
Catalog No.:BCC4414
CAS No.:168273-06-1
- 3,5-Dimethoxy-3'-hydroxybibenzyl
Catalog No.:BCN8112
CAS No.:168281-05-8
- C-Veratroylglycol
Catalog No.:BCN1102
CAS No.:168293-10-5
- 3-O-Acetyl-16 alpha-hydroxytrametenolic acid
Catalog No.:BCN1532
CAS No.:168293-13-8
- 3-O-Acetyl-16 alpha-hydroxydehydrotrametenolic acid
Catalog No.:BCN1531
CAS No.:168293-14-9
- 3-Epidehydropachymic acid
Catalog No.:BCN3644
CAS No.:168293-15-0
- Asiaticoside
Catalog No.:BCN1011
CAS No.:16830-15-2
- Oxymatrine
Catalog No.:BCN1103
CAS No.:16837-52-8
- Tacrine hydrochloride
Catalog No.:BCC6869
CAS No.:1684-40-8
- Compound 401
Catalog No.:BCC7622
CAS No.:168425-64-7
- Rhodiocyanoside A
Catalog No.:BCN7852
CAS No.:168433-86-1
- Epifriedelanol
Catalog No.:BCN1104
CAS No.:16844-71-6
[Chemical consitituents from root of Isatis indigotica].[Pubmed:23944031]
Zhongguo Zhong Yao Za Zhi. 2013 Apr;38(8):1172-82.
Thirty-three compounds were isolated from the root decoction of Isatis indigotica by using a combination of various chromatographic techniques including silica gel, macroporous adsorbent resin, Sephadex LH-20, and reversed-phase HPLC. Their structures were elucidated by spectroscopic data as (+)-dehydrovomifoliol (1), (S)-(+)-abscisic acid (2), vomifoliol (3), cyclo (L-Phe-L-Leu) (4), cyclo(L-Phe-L-Tyr) (5), cyclo(L-Tyr-L-Leu) (6), cyclo(L-Pro-L-Tyr) (7), Evofolin B (8), (+)-syringaresinol (9), (-)-(7R,7'R,8S,8'S)-4,4'-dihydroxy-3-methoxy-7,9';7',9-diepoxy-lignan (10), (-)-medioresinol (11), (+) -(7R,7'R,8S,8'S) -neo-olivil (12), (-) -5-methoxyisolariciresinol (13), 1,3-dihydro-2H-indol-2-one (14), isalexin (15), dihydroneoascorbigen (16), indican (17), (-) -(S) -cyanomethyl-3-hydroxyoxindole (18), isoformononetein (19), calycosin (20), stigamast-5-ene-3beta-ol-7-one (21), acetovanillone (22), 3, 5-dimethoxy-4-hydroxyacetophenone (23), dihydroconiferyl alcohol (24), dihyroferulic acid (25), 3-hydroxy-1-(4-hydroxyphenyl) propan-1-one (26), beta-hydroxypropiovanillone (27), 4-aminobenzoic acid (28), 3-(4-hydroxyphenyl) propan-1-ol (29), 4-(2-hydroxyethyl) phenol (30), 2-methoxy-4-vinylphenol (31), pyrocatechol (32), and 4-pentenamide (33). These compounds were isolated from the root of I. indigotica for the first time. In preliminary in vitro assays, compound 19 showed activity against the influenza virus A/Hanfang/359/95 (H3N2), the herpes simplex virus 1 (HSV-1), and Coxsackie virus B3 (Cox-B3), with IC50 values of 2.06, 6.84, and 8.70 micromol x L(-1), respectively, but other compounds were in-active at a concentration of 1.0 x 10 x (-5) mol x L(-1).
Phenolic compounds obtained from stems of Couepia ulei with the potential to induce quinone reductase.[Pubmed:15022717]
Arch Pharm Res. 2004 Feb;27(2):169-72.
Activity-guided fractionation of the EtOAc-soluble extract of the stems of Couepia ulei, using a bioassay based on the induction of quinone reductase (QR) in cultured Hepa 1c1c7 mouse hepatoma cells led to the isolation of two active compounds, a new natural product, erythro-2,3-bis(4-hydroxy-3-methoxyphenyl)-3-ethoxypropan-1-ol (1), and a known compound, evofolin-B (2), along with five inactive compounds all of known structure, viz., betulinic acid, oleanolic acid, pomolic acid, (+/-)-syringaresinol, and ursolic acid. These isolates were identified by analysis of physical and spectral data. Compounds 1 and 2 exhibited QR inducing activity, with observed CD (concentration required to double induction) values of 16.7 and 16.4 microM, respectively.
[Chemical constituents of stems and branches of Adina polycephala].[Pubmed:20707194]
Zhongguo Zhong Yao Za Zhi. 2010 May;35(10):1261-71.
OBJECTIVE: To investigate chemical constituents of the stems and branches of Adina polycephala and their pharmacological activities. METHOD: The constituents were isolated by a combination of various chromatographic techniques including column chromatography on silica gel, Sephadex LH-20, and C-18, as well as reversed-phase HPLC. Structures of the isolates were identified by spectroscopic data analysis. In vitro cytotoxic, anti-inflammatory, anti-oxidant, anti-HIV, neuroprotective and anti-diabetic activities were screened by using cell-based models. RESULT: Twenty-eight constituents were isolated. Their structures were identified as clemochinenoside B (1), kelampayoside A (2), osmanthuside H (3), 4-hydroxy-3-methoxyphenol-beta-D-[6-O-(4-hydroxy-3,5-dimethoxylbenzoate)]-glucopy ranoside (4), and syringic acid beta-D-glucopyranosyl ester (5). Ten iridoidal glycosides: geniposidic acid (6), geniposide (7), 6beta-hydroxygeniposide (8), 6beta-hydroxygeniposide (9), ixoside (10), ixoside 11-methyl ester (11), 11-methyl forsythide (12), 7beta-hydroxysplendoside (13), gardoside (14) and mussaenosidic acid (15), (+) -pinoresinol (16), (+) -medioresinol (17), (+) -syringaresinol (18), (-)-lariciresinol (19), evofolin-B (20), alpha-hydroxyacetovaillone (21), syringic acid (22), vanillin (23), 3, 4, 5-trimethoxyphenol (24), and 2,6-dimethoxy-1, 4-benzoquinone (25), beta-sitosterol (26), mannitol (27), and daucosterol (28). At a concentration of 1.0 x 10(-5) mol x L(-1), these compounds were inactive in the assays, including cytotoxicity against human tumor cell lines (HCT-8, Bel-7402, BGC-823, A549 and A2780), anti-inflammatory activity against the release of beta-glucuronidase in rat polymorphonuclear leukocytes (PMNs) induced by platelet-activating factor (PAF), antioxidant activity in Fe(2+)-cystine-induced rat liver microsomal lipid peroxidation, anti-HIV activity against HIV-1 replication, neuroprotective activity against serum deprivation or glutamate induced neurotoxicity in cultures of PC12 cells, and the inhibitory activity against protein tyrosine phosphatase 1B (PTP1B). CONCLUSION: Compounds 1-20 were obtained from the genus Adina for the first time. The 13C-NMR data of compounds 10 and 11 were reassigned. A further evaluation of pharmacological activity of these compounds is expected.


