SHU 9119MC3 and MC4 antagonist; MC5 partial agonist CAS# 168482-23-3 |
- PHA-665752
Catalog No.:BCC1181
CAS No.:477575-56-7
- (R)-Crizotinib
Catalog No.:BCC1284
CAS No.:877399-52-5
- Tivantinib (ARQ 197)
Catalog No.:BCC3688
CAS No.:905854-02-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 168482-23-3 | SDF | Download SDF |
| PubChem ID | 9898183 | Appearance | Powder |
| Formula | C54H71N15O9 | M.Wt | 1074.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 20 mg/mL (18.62 mM; Need ultrasonic) | ||
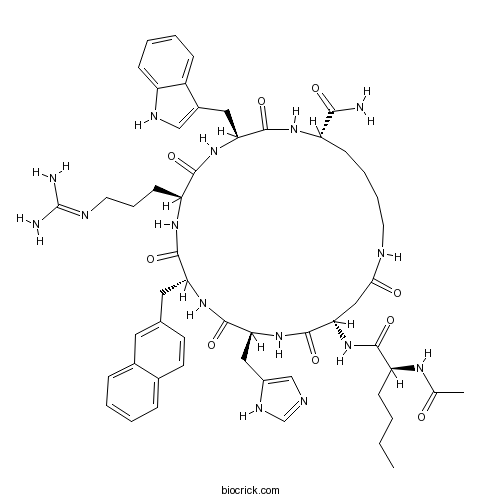
| Sequence | XDHXRWK (Modifications: X-1 = Ac-Nle, X-4 = D-2-Nal, Lys-7 = C-terminal amide, cyclized Asp-2 - Lys-7) | ||
| Chemical Name | (3S,6S,9R,12S,15S,23S)-15-[[(2S)-2-acetamidohexanoyl]amino]-6-[3-(diaminomethylideneamino)propyl]-12-(1H-imidazol-5-ylmethyl)-3-(1H-indol-3-ylmethyl)-9-(naphthalen-2-ylmethyl)-2,5,8,11,14,17-hexaoxo-1,4,7,10,13,18-hexazacyclotricosane-23-carboxamide | ||
| SMILES | CCCCC(C(=O)NC1CC(=O)NCCCCC(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC1=O)CC2=CN=CN2)CC3=CC4=CC=CC=C4C=C3)CCCN=C(N)N)CC5=CNC6=CC=CC=C65)C(=O)N)NC(=O)C | ||
| Standard InChIKey | GGYWLZFXFKFWKL-XOJVSUSESA-N | ||
| Standard InChI | InChI=1S/C54H71N15O9/c1-3-4-15-40(63-31(2)70)48(73)69-45-27-46(71)59-21-10-9-17-39(47(55)72)64-51(76)43(25-35-28-61-38-16-8-7-14-37(35)38)67-49(74)41(18-11-22-60-54(56)57)65-50(75)42(24-32-19-20-33-12-5-6-13-34(33)23-32)66-52(77)44(68-53(45)78)26-36-29-58-30-62-36/h5-8,12-14,16,19-20,23,28-30,39-45,61H,3-4,9-11,15,17-18,21-22,24-27H2,1-2H3,(H2,55,72)(H,58,62)(H,59,71)(H,63,70)(H,64,76)(H,65,75)(H,66,77)(H,67,74)(H,68,78)(H,69,73)(H4,56,57,60)/t39-,40-,41-,42+,43-,44-,45-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent melanocortin MC3 and MC4 receptor antagonist (IC50 values are 0.23 and 0.06 nM respectively) and MC5 partial agonist (EC50 = 0.12 nM). Upregulates expression of genes promoting lipogenesis and triglyceride storage (SCD1, LPL, ACCα and FAS), increases triglyceride synthesis and promotes insulin resistance. Increases food intake, body weight and fat mass when administered centrally in vivo. |

SHU 9119 Dilution Calculator

SHU 9119 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SHU 9119 is a potent human melanocortin 3 and 4 receptors (MC3/4R) antagonist and a partial MC5R agonist; with IC50 values of 0.23, 0.06, and 0.09 nM for human MC3R, MC4R and MC5R, respectively. Sequence: Ac-{Nle}-cyclo[Asp-His-D-{Nal}-Arg-Trp-Lys]-NH2.
In Vivo:Blockade of CNS-Mcr via chronic intracerebroventricular infusion of SHU9119 (24 nmol/d for 7 days) increases food intake in ad libitum-fed rats compared with control. Weight gain of SHU9119 treated rats is significantly higher than control. SHU9119 treatment potently increases metabolic efficiency. SHU9119 markedly increases mRNA levels of genes promoting lipogenesis and TAG storage in adipocytes, including stearoyl-CoA desaturase-1, lipoprotein lipase, acetyl-CoA carboxylase α, and fatty acid synthase[2]. SHU9119 increases food intake (+30%) and body fat (+50%) and decreases EE by reduction in fat oxidation (−42%). In addition, SHU9119 impairs the uptake of VLDL-TG by BAT. In line with this, SHU9119 decreases uncoupling protein-1 levels in BAT (−60%) and induces large intracellular lipid droplets, indicative of severely disturbed BAT activity[3].
References:
[1]. Grieco P, et al. Further structure-activity studies of lactam derivatives of MT-II and SHU-9119: their activity and selectivity at human melanocortin receptors 3, 4, and 5. Peptides. 2007 Jun;28(6):1191-6.
[2]. Nogueiras R, et al. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007 Nov;117(11):3475-88.
[3]. Kooijman S, et al. Inhibition of the central melanocortin system decreases brown adipose tissue activity. J Lipid Res. 2014 Oct;55(10):2022-32.
- Epifriedelanol
Catalog No.:BCN1104
CAS No.:16844-71-6
- Rhodiocyanoside A
Catalog No.:BCN7852
CAS No.:168433-86-1
- Compound 401
Catalog No.:BCC7622
CAS No.:168425-64-7
- Tacrine hydrochloride
Catalog No.:BCC6869
CAS No.:1684-40-8
- Oxymatrine
Catalog No.:BCN1103
CAS No.:16837-52-8
- Asiaticoside
Catalog No.:BCN1011
CAS No.:16830-15-2
- 3-Epidehydropachymic acid
Catalog No.:BCN3644
CAS No.:168293-15-0
- 3-O-Acetyl-16 alpha-hydroxydehydrotrametenolic acid
Catalog No.:BCN1531
CAS No.:168293-14-9
- 3-O-Acetyl-16 alpha-hydroxytrametenolic acid
Catalog No.:BCN1532
CAS No.:168293-13-8
- C-Veratroylglycol
Catalog No.:BCN1102
CAS No.:168293-10-5
- 3,5-Dimethoxy-3'-hydroxybibenzyl
Catalog No.:BCN8112
CAS No.:168281-05-8
- Rimonabant
Catalog No.:BCC4414
CAS No.:168273-06-1
- Z-D-Val-OH
Catalog No.:BCC2732
CAS No.:1685-33-2
- Dehydrogeijerin
Catalog No.:BCN7531
CAS No.:16850-91-2
- Fosbretabulin (Combretastatin A4 Phosphate (CA4P)) Disodium
Catalog No.:BCC4600
CAS No.:168555-66-6
- H-Asp(OMe)-OH.HCl
Catalog No.:BCC2889
CAS No.:16856-13-6
- AIDA
Catalog No.:BCC6841
CAS No.:168560-79-0
- TPEN
Catalog No.:BCC7913
CAS No.:16858-02-9
- Conivaptan HCl
Catalog No.:BCC3756
CAS No.:168626-94-6
- Ezatiostat
Catalog No.:BCC3638
CAS No.:168682-53-9
- H-Tyr-OtBu
Catalog No.:BCC3128
CAS No.:16874-12-7
- SGC707
Catalog No.:BCC6543
CAS No.:1687736-54-4
- Z-Tyr(tBu)-OH.DCHA
Catalog No.:BCC2745
CAS No.:16879-90-6
- RS 67333 hydrochloride
Catalog No.:BCC5693
CAS No.:168986-60-5
Extensive structure-activity studies of lactam derivatives of MT-II and SHU-9119: their activity and selectivity at human melanocortin receptors 3, 4, and 5.[Pubmed:14531843]
J Pept Res. 2003 Nov;62(5):199-206.
The melanocortin system is involved in the regulation of several diverse physiologic pathways. Recently we have demonstrated that replacing His6 by Pro6 in the well-known antagonist SHU-9119 resulted in a potent agonist at the hMC5R (EC50 = 0.072 nm) with full antagonist activity at the hMC3R and the hMC4R. We have designed, synthesized, and pharmacologically characterized a series of peptide analogs of MT-II and SHU-9119 at the human melanocortin receptors MC3R, MC4R and MC5R. All these peptides were modified at position 6 with a Pro instead of a His residue. In this study, we have identified new scaffolds which are antagonists at the hMC4R and hMC3R. Additionally, we have discovered a new selective agonist at the hMC4R, Ac-Nle-c[Asp-Pro-D-Phe-Arg-Trp-Lys]-Pro-Val-NH2 (6, PG-931) which will be useful in further biologic investigations of the hMC4R. PG-931 was about 100-fold more selective for the hMC4R vs. the hMC3R (IC50 = 0.58 and 55 nm, respectively). Some of these new analogs have exceptional biologic potencies at the hMC5R and will be useful in further efforts to differentiate the substructural features responsible for selectivity at the hMC3R, hMC4R, and hMC5R.
Further structure-activity studies of lactam derivatives of MT-II and SHU-9119: their activity and selectivity at human melanocortin receptors 3, 4, and 5.[Pubmed:17482720]
Peptides. 2007 Jun;28(6):1191-6.
Recently we have demonstrated that replacing His(6) by constrained amino acids(2) in the well-known antagonist SHU-9119 resulted in potent and selective antagonist ligands especially at the hMC3R and hMC5 receptors. With the aim to further explore position 6 in the sequence of SHU-9119 and MT-II, we have designed, synthesized, and pharmacologically characterized a series of peptide analogues of MT-II and SHU-9119 at the human melanocortin receptors subtypes MC3R, MC4R and MC5R. All these peptides were modified at position 6 with constrained amino acids which are commercially available. In this study, we have identified new selective ligands for the hMC4R, and an antagonist for the hMC3/hMC4 receptors. Additionally, we have discovered an interesting new selective antagonist at the hMC3R, Ac-Nle-c[Asp-betaAla-DNal(2')-Arg-Trp-Lys]-NH(2) (2, PG-106) which represents an important tool in further biological investigations of the hMC3R. PG-106 will be useful in further efforts to differentiate the substructural features responsible for selectivity at the hMC3R, hMC4R, and hMC5R.
The central melanocortin system directly controls peripheral lipid metabolism.[Pubmed:17885689]
J Clin Invest. 2007 Nov;117(11):3475-88.
Disruptions of the melanocortin signaling system have been linked to obesity. We investigated a possible role of the central nervous melanocortin system (CNS-Mcr) in the control of adiposity through effects on nutrient partitioning and cellular lipid metabolism independent of nutrient intake. We report that pharmacological inhibition of melanocortin receptors (Mcr) in rats and genetic disruption of Mc4r in mice directly and potently promoted lipid uptake, triglyceride synthesis, and fat accumulation in white adipose tissue (WAT), while increased CNS-Mcr signaling triggered lipid mobilization. These effects were independent of food intake and preceded changes in adiposity. In addition, decreased CNS-Mcr signaling promoted increased insulin sensitivity and glucose uptake in WAT while decreasing glucose utilization in muscle and brown adipose tissue. Such CNS control of peripheral nutrient partitioning depended on sympathetic nervous system function and was enhanced by synergistic effects on liver triglyceride synthesis. Our findings offer an explanation for enhanced adiposity resulting from decreased melanocortin signaling, even in the absence of hyperphagia, and are consistent with feeding-independent changes in substrate utilization as reflected by respiratory quotient, which is increased with chronic Mcr blockade in rodents and in humans with loss-of-function mutations in MC4R. We also reveal molecular underpinnings for direct control of the CNS-Mcr over lipid metabolism. These results suggest ways to design more efficient pharmacological methods for controlling adiposity.
Cyclic lactam alpha-melanotropin analogues of Ac-Nle4-cyclo[Asp5, D-Phe7,Lys10] alpha-melanocyte-stimulating hormone-(4-10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors.[Pubmed:7658432]
J Med Chem. 1995 Sep 1;38(18):3454-61.
The cloning of the melanocyte-stimulating hormone (MSH) and adrenocorticotropic hormone (ACTH) receptors (MC1-R and MC2-R, respectively) recently has led to the identification of three additional melanocortin receptors, MC3-R, MC4-R, and MC5-R. The MC2 receptor primarily recognizes only ACTH peptides, but the other four receptors all recognize alpha-melanocyte-stimulating hormone (alpha-MSH) and potent alpha-MSH agonists such as [Nle4,D-Phe7]alpha-MSH-NH2 and Ac-Nle4-c[Asp5,D-Phe7,Lys10]alpha-MSH-(4-10)-NH2 as well as ACTH. The absence of any known physiological role for these new receptors, expressed both in the brain (MC3-R and MC4-R) and throughout a number of peripheral tissues (MC5-R), has necessitated as search for potent and receptor selective agonists and antagonists. We report here that analogues of the superpotent cyclic agonist analogue Ac-Nle4-c[Asp5,D-Phe7, Lys10]alpha-MSH-(4-10)-NH2, in which a bulky aromatic amino acid is substituted in the 7-position, can produce potent and selective antagonists for melanocortin receptors. Thus, the D-p-iodophenylalanine7-containing analogue Ac-Nle4-c[Asp5,D-Phe(pI)7,Lys10]alpha-MSH-(4-10)-NH2 is a potent antagonist (pA2 = 10.3) in the classical frog skin (Rana pipiens) assay (MC1-R), as is the D-2'-naphthylalanine7 (D-Nal(2)7)-containing analogue Ac-Nle4-c[Asp5,D-Nal(2)7,Lys10]alpha-MSH-(4-10)-NH2 (pA2 > 10.3). Interestingly, the D-p-chloro- and D-p-fluorophenylalanine7-containing analogues lacked antagonist activities at all melanotropin receptors, and both exhibited full agonist potency in the frog skin assay. The activity of these analogues also was examined at four mammalian melanocortin receptors. Interestingly, Ac-Nle4-c[Asp5,(D-Nal(2)7,Lys10] alpha-MSH-(4-10)-NH2 was found to be a potent antagonist of the MC4-R (pA2 = 9.3) with minimal agonist activity, a less potent antagonist of the MC3-R (pA2 = 8.3) with minimal agonist activity, and a full agonist of the MC1 and MC5 receptors. Surprisingly, Nle4-c[Asp5,D-Phe(pI)7,Lys10]alpha-MSH was found to be a potent agonist at the cloned human MC1-R (EC50 = 0.055 nM) and mouse MC1-R (EC50 = 0.19 nM) but had potent antagonist activities at the human MC4-R (pA2 = 9.7) and human MC3-R (pA2 = 8.3) with significant partial agonist activities (EC50 = 0.57 and 0.68 nM, respectively) as well. Thus, highly potent and receptor selective antagonist analogues can arise from substitution of the D-Phe7 residue with a bulky aromatic amino acid. These analogues can be used to help determine the functional roles of these receptors.


