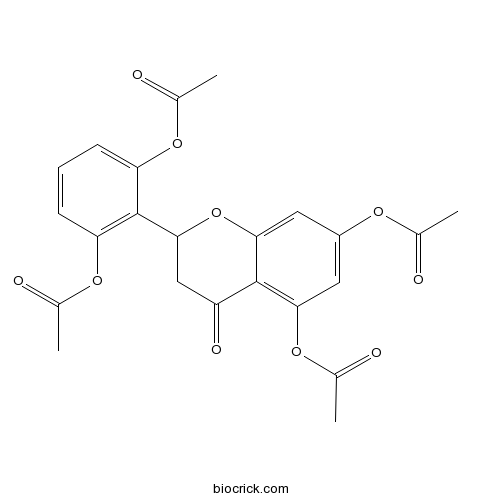
2',5,6',7-TetraacetoxyflavanoneCAS# 80604-17-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 80604-17-7 | SDF | Download SDF |
| PubChem ID | 71307297 | Appearance | Powder |
| Formula | C23H20O10 | M.Wt | 456.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [3-acetyloxy-2-(5,7-diacetyloxy-4-oxo-2,3-dihydrochromen-2-yl)phenyl] acetate | ||
| SMILES | CC(=O)OC1=C(C(=CC=C1)OC(=O)C)C2CC(=O)C3=C(C=C(C=C3O2)OC(=O)C)OC(=O)C | ||
| Standard InChIKey | QAJSRSKXTOZULY-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2',5,6',7-Tetraacetoxyflavanone Dilution Calculator

2',5,6',7-Tetraacetoxyflavanone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1911 mL | 10.9553 mL | 21.9106 mL | 43.8212 mL | 54.7765 mL |
| 5 mM | 0.4382 mL | 2.1911 mL | 4.3821 mL | 8.7642 mL | 10.9553 mL |
| 10 mM | 0.2191 mL | 1.0955 mL | 2.1911 mL | 4.3821 mL | 5.4777 mL |
| 50 mM | 0.0438 mL | 0.2191 mL | 0.4382 mL | 0.8764 mL | 1.0955 mL |
| 100 mM | 0.0219 mL | 0.1096 mL | 0.2191 mL | 0.4382 mL | 0.5478 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2',5,6',7-Tetrahydroxyflavanone
Catalog No.:BCN4345
CAS No.:80604-16-6
- Vitamin B6
Catalog No.:BCN8345
CAS No.:8059-24-3
- Balsalazide
Catalog No.:BCC5225
CAS No.:80573-04-2
- Grifolic acid
Catalog No.:BCN4344
CAS No.:80557-12-6
- Schinifoline
Catalog No.:BCN4343
CAS No.:80554-58-1
- Vitexin 2''-O-(4'''-O-acetyl)rhamnoside
Catalog No.:BCN6740
CAS No.:80537-98-0
- PHTPP
Catalog No.:BCC7447
CAS No.:805239-56-9
- 7beta-(3-Ethyl-cis-crotonoyloxy)-1alpha-(2-methylbutyryloxy)-3,14-dehydro-Z-notonipetranone
Catalog No.:BCN7638
CAS No.:80514-14-3
- Cis-N-Feruloyltyramine
Catalog No.:BCN3729
CAS No.:80510-09-4
- Grossamide
Catalog No.:BCN4571
CAS No.:80510-06-1
- Euchrestaflavanone A
Catalog No.:BCN3576
CAS No.:80510-05-0
- Veratrine
Catalog No.:BCN8444
CAS No.:8051-02-3
- 8-Demethylsideroxylin
Catalog No.:BCN3117
CAS No.:80621-54-1
- Rifaximin (Xifaxan)
Catalog No.:BCC3848
CAS No.:80621-81-4
- Sanggenone C
Catalog No.:BCN6350
CAS No.:80651-76-9
- Fuziline
Catalog No.:BCN2822
CAS No.:80665-72-1
- Dihydroergotoxine mesylate
Catalog No.:BCC6671
CAS No.:8067-24-1
- 5-O-methylvisamminol
Catalog No.:BCC8108
CAS No.:80681-42-1
- Sec-O-Glucosylhamaudol
Catalog No.:BCN1233
CAS No.:80681-44-3
- Prim-O-glucosylcimifugin
Catalog No.:BCN4952
CAS No.:80681-45-4
- 4'-Demethoxypiperlotine C
Catalog No.:BCN6495
CAS No.:807372-38-9
- Z-Arg(Mtr)-OH.CHA
Catalog No.:BCC3062
CAS No.:80745-09-1
- H-Arg(Mtr)-OH.1/2H2O
Catalog No.:BCC2863
CAS No.:80745-10-4
- S-2-Benzothiazolyl2-amino-alpha-(methoxyimino)-4-thiazolethiolacetate
Catalog No.:BCC9138
CAS No.:80756-85-0
Synthesis and Screening of Modified 6,6'-Bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrobenzo[e][1,2,4]triazin-3-yl)-2,2'- bipyridine Ligands for Actinide and Lanthanide Separation in Nuclear Waste Treatment.[Pubmed:27463244]
J Org Chem. 2016 Nov 4;81(21):10517-10520.
Effects of chloro and bromo substitution at the 4-position of the pyridine ring of 6,6'-bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrobenzo[e][1,2,4]triazin-3-yl)-2,2'- bipyridine (CyMe4-BTBP) have been studied with regard to the extraction of Am(III) from Eu(III) and Cm(III) from 0.1-3 M HNO3. Similarly to CyMe4-BTBP, a highly efficient (DAm > 10 at 3 M HNO3) and selective (SFAm/Eu > 100 at 3 M HNO3) extraction was observed for Cl-CyMe4-BTBP and Br-CyMe4-BTBP in 1-octanol but in the absence of a phase-transfer agent.
Crystal structure of poly[di-chlorido-(mu-2,5-di-carb-oxy-benzene-1,4-di-carboxyl-ato-kappa(2)O(1):O(4 ))bis-[mu-4'-(pyridin-3-yl)-4,2':6',4''-terpyridine-kappa(2)N(1):N(4')]dizinc].[Pubmed:27840732]
Acta Crystallogr E Crystallogr Commun. 2016 Oct 28;72(Pt 11):1663-1665.
In the title polymeric Zn(II) complex, [Zn2(C10H4O8)Cl2(C20H14N4)2] n , the Zn(II) cations are bridged by both 2,5-di-carb-oxy-benzene-1,4-di-carboxyl-ate dianions and 4'-(pyridin-3-yl)-4,2':6',4''-terpyridine ligands, forming ladder-like polymeric chains propagating along [1-10]. The Cl(-) anion further coordinates the Zn(II) cation to complete a distorted tetra-hedral environment. In the 4'-(pyridin-3-yl)-4,2':6',4''-terpyridine ligand, the three sideward pyridine rings are twisted with respect to the central pyridine ring by 39.27 (12), 14.89 (13) and 3.36 (13) degrees , respectively. In the crystal, classical O-Hcdots, three dots, centeredN hydrogen bonds and weak C-Hcdots, three dots, centeredO and C-Hcdots, three dots, centeredCl hydrogen bonds link the chains into a three-dimensional supra-molecular architecture. pi-pi stacking is observed between the pyridine and benzene rings of neighbouring polymeric chains, with a centroid-to-centroid distance of 3.7280 (14) A.
Crystal structures of 2'-benzoyl-1'-(4-methyl-phenyl)-1,1',2,2',5',6',7',7a'-octa-hydro-spiro-[indole-3 ,3'-pyrrolizin]-2-one and 2'-(4-bromo-benzoyl)-1'-(2-chloro-phen-yl)-1,1',2,2',5',6',7',7a'-octa-hydro-spir o-[indole-3,3'-pyrrolizin]-2-one.[Pubmed:27840725]
Acta Crystallogr E Crystallogr Commun. 2016 Oct 25;72(Pt 11):1637-1641.
The two title compounds, C28H26N2O2, (I), and C27H22BrClN2O2, (II), differ in their substituents, viz.4-methyl-phenyl and benzoyl rings in (I) replaced by 2-chloro-phenyl and 4- bromo-benzoyl, respectively, in (II). A significant difference between the two mol-ecules is found in the deviation of the benzoyl O atom from the least-squares plane of the ring to which it is attached [0.593 (4) and 0.131 (3) A, respectively], a fact which may be attributed to the different participation of the benzoyl O atoms as acceptors in their inter-molecular C-Hcdots, three dots, centeredO inter-actions. The chemical modifications in (I) and (II) do not seem to affect the type nor strength of the inter-molecular N-Hcdots, three dots, centeredN and C-Hcdots, three dots, centeredO hydrogen bonds responsible for the two crystal structures, such that the aggregation of mol-ecules appears similar in spite of the mol-ecular changes.
Crystal structure of a rare trigonal bipyramidal titanium(IV) coordination complex: tri-chlorido-(3,3'-di-tert-butyl-2'-hy-droxy-5,5',6,6'-tetra-methyl-1,1'-biphenyl -2-olato-kappaO(2))(tetra-hydro-furan-kappaO)-titanium(IV).[Pubmed:28083144]
Acta Crystallogr E Crystallogr Commun. 2017 Jan 1;73(Pt 1):88-91.
The title compound, [Ti(C24H33O2)Cl3(C4H8O)], is a rare example of a trigonal-bipyramidal titanium coordination complex with three chloride and two oxygen donor ligands. The asymmetric unit contains two independent mol-ecules having essentially the same conformation. The mol-ecules feature the titanium(IV) metal cation complexed with three chloride ligands, a tetra-hydro-furan mol-ecule, and one oxygen atom from the resolved ligand precursor (R)-(+)-5,5',6,6'-tetra-methyl-3,3'-di-t-butyl-1,1'-biphenyl-2,2'-diol, where the remaining phenolic hydrogen atom engages in inter-molecular O-Hcdots, three dots, centeredCl hydrogen bonding. In one mol-ecule, the THF ligand is disordered over two orientations with refined site occupancies of 0.50 (3).


