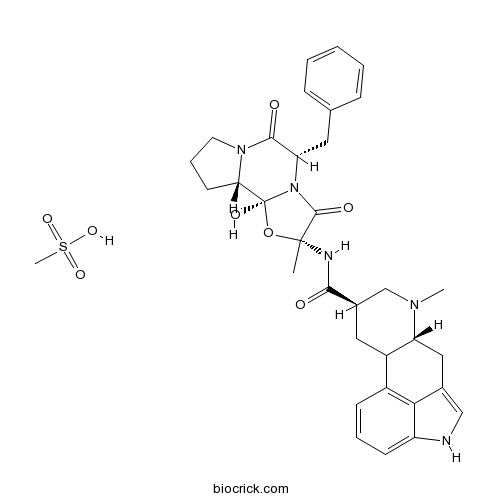
Dihydroergotoxine mesylateBinds to GABAA receptor Cl- channel; allosteric modulator of benzodiazepine site CAS# 8067-24-1 |
- MLN9708
Catalog No.:BCC2091
CAS No.:1201902-80-8
- MG-115
Catalog No.:BCC1237
CAS No.:133407-86-0
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- CEP-18770
Catalog No.:BCC2093
CAS No.:847499-27-8
- Carfilzomib (PR-171)
Catalog No.:BCC1145
CAS No.:868540-17-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 8067-24-1 | SDF | Download SDF |
| PubChem ID | 6420006 | Appearance | Powder |
| Formula | C34H41N5O8S | M.Wt | 679.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ergoloid mesylates | ||
| Solubility | DMSO : ≥ 100 mg/mL (43.20 mM) H2O : 2 mg/mL (0.86 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| SMILES | CC1(C(=O)N2C(C(=O)N3CCCC3C2(O1)O)CC4=CC=CC=C4)NC(=O)C5CC6C(CC7=CNC8=CC=CC6=C78)N(C5)C.CS(=O)(=O)O | ||
| Standard InChIKey | ADYPXRFPBQGGAH-WVVAGBSPSA-N | ||
| Standard InChI | InChI=1S/C33H37N5O5.CH4O3S/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32;1-5(2,3)4/h3-6,8-11,17,21,23,25-27,34,42H,7,12-16,18H2,1-2H3,(H,35,39);1H3,(H,2,3,4)/t21-,23?,25-,26+,27+,32-,33+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A complex of closely related alkaloid salts. Binds with high affinity to the GABAA receptor Cl- channel, producing an allosteric interaction with the benzodiazepine site. Also interacts with central dopaminergic, serotonergic and adrenergic (α1) receptors. Displays antiproliferative activity in vitro (IC50 = 18 - 38 μM in prostate cancer cells) and exhibits cognition-enhancing, anticonvulsant and sedative activity in vivo. Orally active. |

Dihydroergotoxine mesylate Dilution Calculator

Dihydroergotoxine mesylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.471 mL | 7.3551 mL | 14.7102 mL | 29.4204 mL | 36.7755 mL |
| 5 mM | 0.2942 mL | 1.471 mL | 2.942 mL | 5.8841 mL | 7.3551 mL |
| 10 mM | 0.1471 mL | 0.7355 mL | 1.471 mL | 2.942 mL | 3.6776 mL |
| 50 mM | 0.0294 mL | 0.1471 mL | 0.2942 mL | 0.5884 mL | 0.7355 mL |
| 100 mM | 0.0147 mL | 0.0736 mL | 0.1471 mL | 0.2942 mL | 0.3678 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fuziline
Catalog No.:BCN2822
CAS No.:80665-72-1
- Sanggenone C
Catalog No.:BCN6350
CAS No.:80651-76-9
- Rifaximin (Xifaxan)
Catalog No.:BCC3848
CAS No.:80621-81-4
- 8-Demethylsideroxylin
Catalog No.:BCN3117
CAS No.:80621-54-1
- 2',5,6',7-Tetraacetoxyflavanone
Catalog No.:BCN4346
CAS No.:80604-17-7
- 2',5,6',7-Tetrahydroxyflavanone
Catalog No.:BCN4345
CAS No.:80604-16-6
- Vitamin B6
Catalog No.:BCN8345
CAS No.:8059-24-3
- Balsalazide
Catalog No.:BCC5225
CAS No.:80573-04-2
- Grifolic acid
Catalog No.:BCN4344
CAS No.:80557-12-6
- Schinifoline
Catalog No.:BCN4343
CAS No.:80554-58-1
- Vitexin 2''-O-(4'''-O-acetyl)rhamnoside
Catalog No.:BCN6740
CAS No.:80537-98-0
- PHTPP
Catalog No.:BCC7447
CAS No.:805239-56-9
- 5-O-methylvisamminol
Catalog No.:BCC8108
CAS No.:80681-42-1
- Sec-O-Glucosylhamaudol
Catalog No.:BCN1233
CAS No.:80681-44-3
- Prim-O-glucosylcimifugin
Catalog No.:BCN4952
CAS No.:80681-45-4
- 4'-Demethoxypiperlotine C
Catalog No.:BCN6495
CAS No.:807372-38-9
- Z-Arg(Mtr)-OH.CHA
Catalog No.:BCC3062
CAS No.:80745-09-1
- H-Arg(Mtr)-OH.1/2H2O
Catalog No.:BCC2863
CAS No.:80745-10-4
- S-2-Benzothiazolyl2-amino-alpha-(methoxyimino)-4-thiazolethiolacetate
Catalog No.:BCC9138
CAS No.:80756-85-0
- Tectorigenin sodium sulfonate
Catalog No.:BCN8161
CAS No.:807636-25-5
- 3-Acetylyunaconitine
Catalog No.:BCN7640
CAS No.:80787-51-5
- 1-Hydroxycanthin-6-one
Catalog No.:BCN4347
CAS No.:80787-59-3
- FG-4592 (ASP1517)
Catalog No.:BCC2227
CAS No.:808118-40-3
- Pyracrenic acid
Catalog No.:BCN7455
CAS No.:80832-44-6
Bioavailability of slow-release co-dergocrine mesylate (dihydroergotoxine) formulations.[Pubmed:3436686]
Int J Clin Pharmacol Ther Toxicol. 1987 Dec;25(12):660-3.
The relative bioavailability and plasma concentration profile of dihydroergotoxine as a solution, standard tablets and slow-release formulations have been compared in 12 healthy subjects following the application of 4-5 mg as a single dose or as 2-3 smaller dose units given at 5 h intervals. Plasma dihydroergotoxine concentrations were measured using a sensitive and highly specific radioimmunoassay procedure. The plasma concentration profile after a single 4.5 mg SR-capsule with relative bioavailability 92% (Oral solution, 4.5 mg as single dose = 100%), releasing 80% of the capsule contents within 8 h, was similar to that achieved following the application of 2-3 divided doses at 5 h intervals of a solution (relative bioavailability = 85%), standard tablet (relative bioavailability = 99%) or SR-tablets (relative bioavailability 78%). Slow-release formulations of dihydroergotoxine do not lead to high postdose concentrations as seen with solutions and standard tablets. Application of a single 4.5 mg SR-capsule can maintain prolonged plateau concentrations exceeding 100 pg/ml over 10 h. Reducing the rate of presentation of dihydroergotoxine to the body in the form of slow release formulations or by using spaced doses does not markedly affect bioavailability when compared to a solution.
Effect of dihydroergotoxine mesylate (Hydergine) on delayed neuronal death in the gerbil hippocampus.[Pubmed:2852425]
Acta Neurol Scand. 1988 Sep;78(3):214-20.
The CA 1 neurons in the gerbil hippocampus exhibiting necrosis with delayed onset following 5 min ischemia were reduced markedly by the systemic administration of Dihydroergotoxine mesylate (Hydergine; HYG). Immediately after 5 min of forebrain ischemia, the animals were injected intraperitoneally with HYG. Seven days after ischemia, perfusion-fixed brains were processed by conventional histology. The number of neurons per millimeter in the CA 1 pyramidal cell layer were calculated and they were labelled neuronal density. In the control group, the neuronal density was 66.03 +/- 7.37 (mean +/- SEM), in the vehicle group, it was 11.25 +/- 4.93. The neuronal density in the HYG group was 69.19 +/- 6.49. The difference in the neuronal density between the HYG group and the control group was not statistically significant. These data indicate that HYG protects on the CA 1 neurons, and this suggest that the suppression of adrenoceptors by this drugs may be the main mechanism of action. This morphologic outcome may explain the functional amelioration of mental impairment by HYG.
[Dihydroergotoxine mesylate in the treatment of functioning pituitary adenoma].[Pubmed:2475811]
Neurol Med Chir (Tokyo). 1989 Feb;29(2):94-8.
Although it is well known that bromocriptine (BC) is effective in the treatment of functioning pituitary adenoma, this agent sometimes causes severe gastrointestinal side effects. In this study, Dihydroergotoxine mesylate (EX), which is composed of ergot alkaloids and is similar to BC, was administered to 11 patients with functioning pituitary adenomas who could not tolerate the adverse effects of BC. Three patients (27%) showed clinical improvement with EX treatment alone (1 to 6 mg/day). In another patient, computed tomography demonstrated tumor shrinkage. The remaining seven patients experienced adverse effects while taking EX. These results indicate that EX is a useful alternative to BC in the treatment of functioning pituitary adenoma, particularly in patients who cannot tolerate the side effects of BC. Moreover, pretreatment with EX appears to reduce the incidence of side effects of BC.
Expression of gamma-aminobutyric acid receptor (subtype A) in prostate cancer.[Pubmed:18607852]
Acta Oncol. 2008;47(8):1546-50.
BACKGROUND: In prostate cancer, gamma-aminobutyric acid (GABA) has been previously reported to increase cellular proliferation via the ionotropic GABAa receptor (GABAar) and to promote cellular invasiveness via the metabotropic GABAb receptor. METHODS: In this study, we have investigated, by immunohistochemistry, GABAar levels in 12 normal human prostate, 13 benign prostatic hyperplasia (BPH) and 148 human prostate cancer specimens. We have also examined the effect of several GABA agonists and antagonists on the in vitro proliferation of four human prostate cancer cell lines: LNCaP, MDA-PCA-2b, DU145 and PC3. RESULTS: GABAar immunoreactivity was present in the stroma of ~75% of the normal and BPH specimens, and in 95% of the prostate cancer specimens. Also, low to moderate GABAar staining was observed in the acinar epithelium of 50 (33%) prostate cancer specimens. No correlation was observed between GABAar staining and patient age, Gleason Sum or TNM stage. A GABAa agonist isoguvacine, at doses between 5-50 microg/ml (31-310 microM), stimulated the proliferation of all four human prostate cancer cell lines, tested. Baclofen, a GABAb agonist (up to 50 microg/ml, 234 microM) had no effect on growth. Also, at concentrations up to 100 microg/ml, GABA antagonists, bicuculline (223 microM), picrotoxin (166 microM) and saclofen (400 microM), did not have significant growth-inhibitory effects. However, dihydroergotoxine, which binds the GABAar chloride ion-channel, inhibited cellular proliferation (IC(50) 18-38 microM). CONCLUSIONS: These data indicate frequent expression of GABAar in prostate cancer and support a role for GABAar in the proliferation of prostate cancer cells.
Effect of ergot alkaloids on 3H-flunitrazepam binding to mouse brain GABAA receptors.[Pubmed:12955907]
Coll Antropol. 2003;27 Suppl 1:175-82.
In vitro effects of dihydroergotoxine, dihydroergosine, dihydroergotamine, alpha-dihydroergocriptine (ergot alkaloids), diazepam, methyl-beta-Carboline-3-carboxilate (beta-CCM), flumazenil (benzodiazepines), gamma-amino butyric acid (GABA) and thiopental (barbiturate) were studied on mouse brain (cerebrum minus cerebral cortex) benzodiazepine binding sites labeled with 3H-flunitrazepam. Specific, high affinity (affinity constant, Kd = 57.7 8.6 nM) binding sites for 3H-flunitrazepam on mouse brain membranes were identified. All benzodiazepine drugs inhibited 3H-flunitrazepam binding with nanomolar potencies. In contrast to benzodiazepines, all ergot drugs, GABA and thiopental produced an enhancement of 3H-flunitrazepam binding to its binding site at the GABAA receptor of the mouse brain. The rank order of potency was: neurotransmitter (GABA) > dihydroergotoxine > thiopental > alpha-dihydroergocriptine > dihydroergosine > dihydroergotamine. The results suggest that dihydrogenated ergot derivatives do not bind to the brain benzodiazepine binding sites labeled with 3H-flunitrazepam. However, an enhancement of 3H-flunitrazepam binding by all ergot drugs tested, clearly identifies an allosteric interaction with the benzodiazepine binding sites of GABAA receptors.
Dihydroergotoxine modulation of the GABAA receptor-associated Cl- ionophore in mouse brain.[Pubmed:1333969]
Eur J Pharmacol. 1992 Oct 6;221(1):139-43.
Dihydroergotoxine non-competitively displaced the binding of t-[3H]butylbicycloorthobenzoate ([3H]TBOB) to crude synaptosomal membranes from the mouse brain (cerebrum minus cortex), and gamma-aminobutyric acid (GABA) (10 microM) enhanced the displacement potency of dihydroergotoxine in a bicuculline-sensitive manner. The same ergot compound prolonged pentobarbital-induced sleeping in mice and diminished the convulsive potency of picrotoxin in the same animal species. The results are indicative of the positive coupling between GABA and dihydroergotoxine.
Dihydrogenated ergot compounds bind with high affinity to GABAA receptor-associated Cl- ionophore.[Pubmed:1664802]
Eur J Pharmacol. 1991 Sep 4;202(1):109-11.
The binding of t-[3H]butylbicycloorthobenzoate ([3H]TBOB) to crude synaptosomal membranes of the mouse brain (cerebrum minus cortex) in the presence of dihydroergotoxine, dihydroergosine, dihydroergotamine and gamma-aminobutyric acid (GABA) was studied in vitro. [3H]TBOB binding was inhibited by all drugs used. The rank order of potency was dihydroergotoxine greater than GABA greater than dihydroergosine greater than dihydroergotamine. This suggests that dihydrogenated ergot compounds, especially dihydroergotoxine, possess appreciable binding activity (comparable to that of benzodiazepines and barbiturates) at the GABAA receptor-associated C1- ionophore.


