Euchrestaflavanone ACAS# 80510-05-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 80510-05-0 | SDF | Download SDF |
| PubChem ID | 484588 | Appearance | Powder |
| Formula | C25H28O5 | M.Wt | 408.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
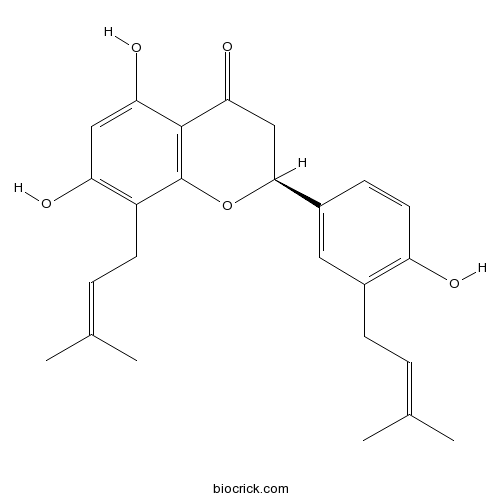
| Chemical Name | (2S)-5,7-dihydroxy-2-[4-hydroxy-3-(3-methylbut-2-enyl)phenyl]-8-(3-methylbut-2-enyl)-2,3-dihydrochromen-4-one | ||
| SMILES | CC(=CCC1=C(C=CC(=C1)C2CC(=O)C3=C(O2)C(=C(C=C3O)O)CC=C(C)C)O)C | ||
| Standard InChIKey | BMIMEYWWZBBDCM-QHCPKHFHSA-N | ||
| Standard InChI | InChI=1S/C25H28O5/c1-14(2)5-7-16-11-17(8-10-19(16)26)23-13-22(29)24-21(28)12-20(27)18(25(24)30-23)9-6-15(3)4/h5-6,8,10-12,23,26-28H,7,9,13H2,1-4H3/t23-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Euchrestaflavanone A reveals potent cytotoxicities against one or more cell lines with IC 50 values in the range of 4.5–9.961μM. 2. Euchrestaflavanone A is an inhibitor of MRP1-like efflux activity in human erythrocytes. |

Euchrestaflavanone A Dilution Calculator

Euchrestaflavanone A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.448 mL | 12.2399 mL | 24.4798 mL | 48.9596 mL | 61.1995 mL |
| 5 mM | 0.4896 mL | 2.448 mL | 4.896 mL | 9.7919 mL | 12.2399 mL |
| 10 mM | 0.2448 mL | 1.224 mL | 2.448 mL | 4.896 mL | 6.12 mL |
| 50 mM | 0.049 mL | 0.2448 mL | 0.4896 mL | 0.9792 mL | 1.224 mL |
| 100 mM | 0.0245 mL | 0.1224 mL | 0.2448 mL | 0.4896 mL | 0.612 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Veratrine
Catalog No.:BCN8444
CAS No.:8051-02-3
- Glaucocalyxin B
Catalog No.:BCN8440
CAS No.:80508-81-2
- Tenacigenin B
Catalog No.:BCN4342
CAS No.:80508-42-5
- Mezlocillin Sodium Monohydrate
Catalog No.:BCC5634
CAS No.:80495-46-1
- Rubifolic acid
Catalog No.:BCN4341
CAS No.:80489-65-2
- Fluticasone propionate
Catalog No.:BCC4907
CAS No.:80474-14-2
- Sapogenins Glycosides
Catalog No.:BCC5320
CAS No.:8047-15-2
- Paeoniflorigenone
Catalog No.:BCN3933
CAS No.:80454-42-8
- Padmatin
Catalog No.:BCN4340
CAS No.:80453-44-7
- Dynorphin A
Catalog No.:BCC7596
CAS No.:80448-90-4
- gamma-Diasarone
Catalog No.:BCN4339
CAS No.:80434-33-9
- Calcium Levofolinate
Catalog No.:BCC4643
CAS No.:80433-71-2
- Grossamide
Catalog No.:BCN4571
CAS No.:80510-06-1
- Cis-N-Feruloyltyramine
Catalog No.:BCN3729
CAS No.:80510-09-4
- 7beta-(3-Ethyl-cis-crotonoyloxy)-1alpha-(2-methylbutyryloxy)-3,14-dehydro-Z-notonipetranone
Catalog No.:BCN7638
CAS No.:80514-14-3
- PHTPP
Catalog No.:BCC7447
CAS No.:805239-56-9
- Vitexin 2''-O-(4'''-O-acetyl)rhamnoside
Catalog No.:BCN6740
CAS No.:80537-98-0
- Schinifoline
Catalog No.:BCN4343
CAS No.:80554-58-1
- Grifolic acid
Catalog No.:BCN4344
CAS No.:80557-12-6
- Balsalazide
Catalog No.:BCC5225
CAS No.:80573-04-2
- Vitamin B6
Catalog No.:BCN8345
CAS No.:8059-24-3
- 2',5,6',7-Tetrahydroxyflavanone
Catalog No.:BCN4345
CAS No.:80604-16-6
- 2',5,6',7-Tetraacetoxyflavanone
Catalog No.:BCN4346
CAS No.:80604-17-7
- 8-Demethylsideroxylin
Catalog No.:BCN3117
CAS No.:80621-54-1
Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities.[Pubmed:24443427]
Chem Biodivers. 2014 Jan;11(1):73-84.
A new limonoid, 7-O-acetyl-7-O-debenzoyl-22-hydroxy-21-methoxylimocinin (2), and two new flavonoids, 3'-(3-hydroxy-3-methylbutyl)naringenin (7) and 4'-O-methyllespedezaflavanone C (9), along with nine known compounds, including two limonoids, 1 and 3, and seven flavonoids, 4-6, 8, and 10-12, were isolated from a MeOH extract of the flowers of Azadirachta indica A.Juss. var. siamensis Valeton (Siamese neem tree; Meliaceae). The structures of new compounds were elucidated on the basis of extensive spectroscopic analysis and comparison with literature data. All of these compounds were evaluated for their melanogenesis-inhibitory activities in B16 melanoma cells induced with alpha-melanocyte-stimulating hormone (alpha-MSH). Compound 2 (16.9% melanin content at 30 muM), 6-deacetylnimbin (3; 49.6% melanin content at 100 muM), and kaempferide (10; 41.7% melanin content at 10 muM) exhibited inhibitory effects with no, or almost no, toxicity to the cells (81.0-111.7% cell viability). In addition, evaluation of their cytotoxic activities against HL60, A549, AZ521, and SK-BR-3 human cancer cell lines, isoazadironolide (1), 4'-O-methyl-8-prenylnaringenin (5), Euchrestaflavanone A (8), 9, and 3-methoxy-3'-prenylnaringenin (12) revealed potent cytotoxicities against one or more cell lines with IC50 values in the range of 4.5-9.9 muM.
Flavonoids as inhibitors of MRP1-like efflux activity in human erythrocytes. A structure-activity relationship study.[Pubmed:12812360]
Oncol Res. 2003;13(11):463-9.
The potency of flavonoids (isoflavones, flavones, and flavanones) to inhibit efflux of 2',7'-bis-(carboxypropyl)-5(6)-carboxyfluorescein (BCPCF) from human erythrocytes was investigated. Structure-activity relationship analysis showed that the strongest inhibitors were found among flavanones bearing a hydrophobic prenyl, geranyl, or lavandulyl group at position 8 (and hydroxyl groups at 5 and 7) in ring A. A prenyl group at position 5' or stilbene at positions 4'-5' in ring B further seemed to increase inhibitor potency. The most efficient flavanones, Euchrestaflavanone A and sophoraflavanone H, were approximately 20 times more efficient than genistein, and induced 50% inhibition of BCPCF efflux (IC50) at 3 microM (60 min, 37 degrees C). This is comparable to IC50 of benzbromarone (4 microM) and lower than IC50 of indomethacin (10 microM), both known MRP1 (ABCC1) inhibitors. It is suggested that BCPCF efflux is mainly due to MRP1 activity. Our results indicate that flavonoid molecular structure provides a promising base for development of potent MRP1 inhibitors.


