5-O-methylvisamminolCAS# 80681-42-1 |
Quality Control & MSDS
Number of papers citing our products

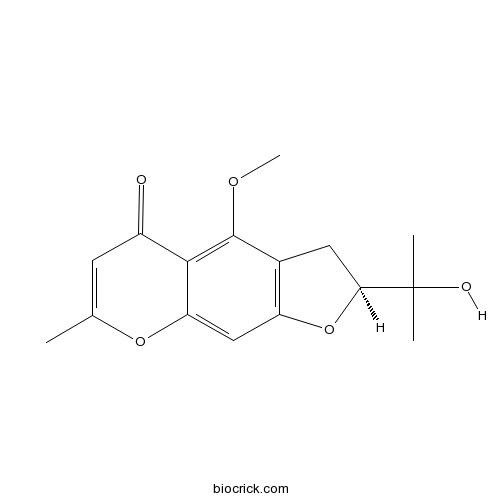
Chemical structure

3D structure
| Cas No. | 80681-42-1 | SDF | Download SDF |
| PubChem ID | 441970 | Appearance | Beige powder |
| Formula | C16H18O5 | M.Wt | 290.3 |
| Type of Compound | Phenylpropanes | Storage | Desiccate at -20°C |
| Solubility | Soluble in chloroform and methan | ||
| Chemical Name | (2S)-2-(2-hydroxypropan-2-yl)-4-methoxy-7-methyl-2,3-dihydrofuro[3,2-g]chromen-5-one | ||
| SMILES | CC1=CC(=O)C2=C(C3=C(C=C2O1)OC(C3)C(C)(C)O)OC | ||
| Standard InChIKey | DGFLRNOCLJGHLY-ZDUSSCGKSA-N | ||
| Standard InChI | InChI=1S/C16H18O5/c1-8-5-10(17)14-12(20-8)7-11-9(15(14)19-4)6-13(21-11)16(2,3)18/h5,7,13,18H,6H2,1-4H3/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

5-O-methylvisamminol Dilution Calculator

5-O-methylvisamminol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4447 mL | 17.2236 mL | 34.4471 mL | 68.8942 mL | 86.1178 mL |
| 5 mM | 0.6889 mL | 3.4447 mL | 6.8894 mL | 13.7788 mL | 17.2236 mL |
| 10 mM | 0.3445 mL | 1.7224 mL | 3.4447 mL | 6.8894 mL | 8.6118 mL |
| 50 mM | 0.0689 mL | 0.3445 mL | 0.6889 mL | 1.3779 mL | 1.7224 mL |
| 100 mM | 0.0344 mL | 0.1722 mL | 0.3445 mL | 0.6889 mL | 0.8612 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dihydroergotoxine mesylate
Catalog No.:BCC6671
CAS No.:8067-24-1
- Fuziline
Catalog No.:BCN2822
CAS No.:80665-72-1
- Sanggenone C
Catalog No.:BCN6350
CAS No.:80651-76-9
- Rifaximin (Xifaxan)
Catalog No.:BCC3848
CAS No.:80621-81-4
- 8-Demethylsideroxylin
Catalog No.:BCN3117
CAS No.:80621-54-1
- 2',5,6',7-Tetraacetoxyflavanone
Catalog No.:BCN4346
CAS No.:80604-17-7
- 2',5,6',7-Tetrahydroxyflavanone
Catalog No.:BCN4345
CAS No.:80604-16-6
- Vitamin B6
Catalog No.:BCN8345
CAS No.:8059-24-3
- Balsalazide
Catalog No.:BCC5225
CAS No.:80573-04-2
- Grifolic acid
Catalog No.:BCN4344
CAS No.:80557-12-6
- Schinifoline
Catalog No.:BCN4343
CAS No.:80554-58-1
- Vitexin 2''-O-(4'''-O-acetyl)rhamnoside
Catalog No.:BCN6740
CAS No.:80537-98-0
- Sec-O-Glucosylhamaudol
Catalog No.:BCN1233
CAS No.:80681-44-3
- Prim-O-glucosylcimifugin
Catalog No.:BCN4952
CAS No.:80681-45-4
- 4'-Demethoxypiperlotine C
Catalog No.:BCN6495
CAS No.:807372-38-9
- Z-Arg(Mtr)-OH.CHA
Catalog No.:BCC3062
CAS No.:80745-09-1
- H-Arg(Mtr)-OH.1/2H2O
Catalog No.:BCC2863
CAS No.:80745-10-4
- S-2-Benzothiazolyl2-amino-alpha-(methoxyimino)-4-thiazolethiolacetate
Catalog No.:BCC9138
CAS No.:80756-85-0
- Tectorigenin sodium sulfonate
Catalog No.:BCN8161
CAS No.:807636-25-5
- 3-Acetylyunaconitine
Catalog No.:BCN7640
CAS No.:80787-51-5
- 1-Hydroxycanthin-6-one
Catalog No.:BCN4347
CAS No.:80787-59-3
- FG-4592 (ASP1517)
Catalog No.:BCC2227
CAS No.:808118-40-3
- Pyracrenic acid
Catalog No.:BCN7455
CAS No.:80832-44-6
- 3,22-Dihydroxyolean-12-en-29-oic acid
Catalog No.:BCN1347
CAS No.:808769-54-2
Simultaneous analysis of seven marker compounds from Saposhnikoviae Radix, Glehniae Radix and Peucedani Japonici Radix by HPLC/PDA.[Pubmed:27016947]
Arch Pharm Res. 2016 May;39(5):695-704.
A new combination of high performance liquid chromatography (HPLC) method coupled with photodiode array (PDA) analysis has been developed for the simultaneous quantitative determination of seven major components in Saposhnikoviae Radix (SR), Glehniae Radix (GR) and Peucedani Japonici Radix (PR), namely peucedanol 7-O-beta-D-glucopyranoside (1), prim-O-glucosylcimifugin (2), cimifugin (3), 4'-O-beta-D-glucosyl-5-O-methylvisamminol (4), bergapten (5), sec-O-glucosylhamaudol (6), and imperatorin (7). Clear separation of these seven components were achieved on a Phenomenex Kinetex C18 (250 x 4.6 mm, 5 mum) column by gradient elution of water (A) and methanol (B) as mobile phase. The flow rate was 1.0 mL/min and the UV detector wavelength was set at 254 nm. The method was successfully used in the analysis of SR, GR, and PR with relatively simple conditions and procedures, and the results were satisfactory for linearity, recovery, precision, accuracy, stability and robustness. The results indicate that the established HPLC/PDA method is suitable for the classification of SR, GR, and PR.
Identification of 4'-O-beta-D-glucosyl-5-O-methylvisamminol as a novel epigenetic suppressor of histone H3 phosphorylation at Ser10 and its interaction with 14-3-3epsilon.[Pubmed:25205188]
Bioorg Med Chem Lett. 2014 Oct 1;24(19):4763-4767.
Natural compounds are regarded as a rich source for potential anti-inflammatory and anti-carcinogenic agents. Increasing evidence indicates that histone phosphorylation at Ser10 is a marker for cell cycle progression during the mitosis and the induction of immediate pro-inflammatory genes during the interphase. In the present study, we have screened our in-house natural compounds to find out new chemical inhibitor(s) of histone H3 phosphorylation at Ser10. As a result, we observed that alpha-amyrin, oleanolic acid, marliolide, and 4'-O-beta-D-glucosyl-5-O-methylvisamminol decreased the levels of histone H3 phosphorylation at Ser10 and c-Jun. In particular, we observed that 4'-O-beta-D-glucosyl-5-O-methylvisamminol suppressed the direct interaction of histone H3 with 14-3-3epsilon, inhibited the aurora B kinase activity and delayed the mitotic cell cycle progression. We reports 4'-O-beta-D-glucosyl-5-O-methylvisamminol as the first epigenetic natural chemical inhibitor that can abrogates the mitotic cell cycle progression and immediate pro-inflammatory gene expressions via suppression of histone H3 phosphorylation at Ser10 and its interaction with 14-3-3epsilon.
4'-O-beta-D-Glucosyl-5-O-Methylvisamminol, A Natural Histone H3 Phosphorylation Epigenetic Suppressor, Exerts a Neuroprotective Effect Through PI3K/Akt Signaling Pathway on Focal Cerebral Ischemia in Rats.[Pubmed:26868427]
World Neurosurg. 2016 May;89:474-88.
BACKGROUND: A bursting inflammation has been observed that compromises neurologic function in patients who experience stroke. We sought to examine the neuroprotective efficacy of 4'-O-beta-D-glucosyl-5-O-methylvisamminol (OGOMV), a novel histone H3 phosphorylation epigenetic suppressor) in a transient middle cerebral artery occlusion (tMCAO). METHODS: A rodent tMCAO model was used. Administration with 400 mug/kg/day OGOMV was initiated 12 hours before (prevention) and 1 hour after animals were subjected to tMCAO (reversal). The cerebral cortex was harvested to examine protein kinase B (PI3D/Akt), 5-bromo-2'-deoxyuridine (Western blot), and caspases (reverse-transcription polymerase chain reaction). In addition, cerebrospinal fluid samples were collected to examine interleukin 1-beta, interleukin-6, monocyte chemoattractant protein-1, and tumor necrosis factor-alpha (reverse-transcription polymerase chain reaction). RESULTS: Cortical 5-bromo-2'-deoxyuridine and phospho-PI3D/Akt were reduced in tMCAO animals, compared with the healthy controls but increased in the OGOMV treatment and prevention groups. Activated cortical caspase-3,-6, and -9a as well as increased IL-1beta and TNF-alpha levels were observed in the tMCAO animals (P < 0.05). Both prevention and treatment with OGOMV significantly reduced cleaved caspase-3 and -9a groups, but no significant change in caspase-6 was noted. Perifosine, an Akt inhibitor, was added to reduce the bioexpression of phospho-P13D/Akt, and Bcl-2 level and increased cleaved caspase-9a level in both OGOMV prevention and treatment tMCAO groups (P > 0.05). CONCLUSION: Our study suggests that OGOMV could exert a neuroprotective effect by inhibiting the P13D/Akt protein, attenuating inflammation, and cleaved caspase-3- and -9a-related apoptosis. This study also lends credence to support the notion that the prevention of OGOMV could attenuate proinflammatory cytokine mRNA and late-onset caspases in tMCAO and merits further study.
Chemical Composition of the Essential Oil and Diethyl Ether Extract of Trinia glauca (L.) Dumort. (Apiaceae) and the Chemotaxonomic Significance of 5-O-Methylvisamminol.[Pubmed:26919331]
Chem Biodivers. 2016 Apr;13(4):403-15.
Analyses by GC, GC/MS, and NMR spectroscopy (1D- and 2D-experiments) of the essential oil and Et2O extract of Trinia glauca (L.) Dumort. (Apiaceae) aerial parts allowed a successful identification of 220 constituents, in total. The major identified compounds of the essential oil were (Z)-falcarinol (10.6%), bicyclogermacrene (8.0%), germacrene D (7.4%), delta-cadinene (4.3%), and beta-caryophyllene (3.2%), whereas (Z)-falcarinol (47.2%), nonacosane (7.4%), and 5-O-methylvisamminol (4.0%) were the dominant constituents of the extract of T. glauca. One significant difference between the compositions of the herein and the previously analyzed T. glauca essential oils (only two reports) was noted. (Z)-Falcarinol was the major constituent in our case, whereas germacrene D (14.4 and 19.6%) was the major component of the previously studied oils. Possible explanations for this discrepancy were discussed. 5-O-methylvisamminol, a (furo)chromone identified in the extract of T. glauca, has a limited occurrence in the plant kingdom and is a possible excellent chemotaxonomic marker (family and/or subfamily level) for Apiaceae.
4'-O-beta-D-glucosyl-5-O-methylvisamminol, an active ingredient of Saposhnikovia divaricata, attenuates high-mobility group box 1 and subarachnoid hemorrhage-induced vasospasm in a rat model.[Pubmed:26395442]
Behav Brain Funct. 2015 Sep 22;11(1):28.
BACKGROUND: High-mobility group box 1 (HMGB1) was observed to be an important extracellular mediator involved in vascular inflammation associated with subarachnoid hemorrhage (SAH). This study is of interest to examine the efficacy of 4'-O-beta-D-glucosyl-5-O-methylvisamminol (4OGOMV), C22H28O10, on the alternation of cytokines and HMGB1 in an animal model. METHODS: A rodent double hemorrhage SAH model was employed. Administration with 4OGOMV was initiated 1 h after animals were subjected to SAH. Basilar arteries (BAs) were harvested and cortexes examined for HMGB1 mRNA, protein expression (Western blot) and monocyte chemoattractant protein-1 (MCP-1) immunostaining. Cerebrospinal fluid samples were collected to examine IL-1beta, IL-6, IL-8 and MCP-1 (rt-PCR). RESULTS: Morphological findings revealed endothelial cell deformity, intravascular elastic lamina torture, and smooth muscle necrosis in the vessels of SAH groups. Correspondently, IL-1beta, IL-6 and MCP-1 in the SAH-only and SAH-plus vehicle groups was also elevated. 4OGOMV dose-dependently reduced HMGB1 protein expression when compared with the SAH groups.(p < 0.01) Likewise, 400 mug/kg 4OGOMV reduced IL-1beta, MCP-1 and HMGB1 mRNA levels as well as MCP-1(+) monocytes when compared with the SAH groups.. CONCLUSION: 4OGOMV exerts its neuro-protective effect partly through the dual effect of inhibiting IL-6 and MCP-1 activation and also reduced HMGB1 protein, mRNA and MCP-1(+) leukocytes translocation. This study lends credence to validating 4OGOMV as able to attenuate pro-inflammatory cytokine mRNA, late-onset inflammasome, and cellular basis in SAH-induced vasospasm.
Feeble Antipyretic, Analgesic, and Anti-inflammatory Activities were Found with Regular Dose 4'-O-beta-D-Glucosyl-5-O-Methylvisamminol, One of the Conventional Marker Compounds for Quality Evaluation of Radix Saposhnikoviae.[Pubmed:28216902]
Pharmacogn Mag. 2017 Jan-Mar;13(49):168-174.
INTRODUCTION: 4'-O-beta-D-glucosyl-5-O-methylvisamminol (GML) is a conventional marker compound for quality control of Radix Saposhnikoviae. Despite that, neither pharmacodynamic or pharmacokinetic information is available with regard to GML. As such, the aim of thisstudy was to assess the conventional evaluation indices for the quality of Radix Saposhnikoviae. MATERIALS AND METHODS: Pyretic animal model, hot plate test, and ear edema model were established to evaluate and compare the antipyretic, analgesic, and anti-inflammatory effect of the chromone derivativescimifugin, prime-O-glucosylcimifugin (PGCN), and GML in Radix Saposhnikoviae. High performance liquid chromatography separation and analysis was used to obtain pharmacokinetic parameters. Simulated gastric fluid and simulated intestinal fluid was used to investigate the metabolite profiles of PGCN and GML in gastrointestinal tract. RESULTS: Cimifugin exerted a marked dose-dependent antipyretic, analgesic, and anti-inflammatory effect, whereas the effects of PGCN were relatively lower. GML had feeble pharmacodynamic effects. Pharmacokinetic study showed that only cimifugin was detected in the plasma sample of cimifugin and PGCN-treated animals, with drug concentration in the former much higher than the latter. No components were traced in the plasma samples from GML-treated rats. Stability study showed that PGCN and GML was predominantly biotransformed into cimifugin and 5-O-methyvisammiol, respectively. The latter was proven to be extremely unstable in liver tissue homogenate and plasma. CONCLUSIONS: A feeble antipyretic, analgesic, and anti-inflammatory activities was observed when GML was orally delivered. Given that Radix Saposhnikoviae extract is generally administered orally, we speculate that this compound might be a nonpharmacolagically active agent in real usage. Thus, it might be unscientific to evaluate the quality of Radix Saposhnikoviae based on the content of GML. SUMMARY: GML-derived cimifugin, which represents the potential pharma codynamic component of Radix Saposhnikoviae chromones, in plasma was almost nil in contrast to cimifugin and PGCN. And thus, feeble antipyretic, analgesic, and anti-inflammatory activities were found with GML. Abbreviations used: AUC:area under concentration-time curve, DNP:2,4-Dinitrophenol, HPLC:high performance liquid chromatography, HPLC-MS:high performance liquid chromatography- mass spectrography, GML:4'-O-beta-D-glucosyl-5-O-methylvisamminol, MVL:5-O-methyvisammiol, PGCN:prime-O-glucosylcimifugin, SGF:alkaline phosphatase. SIF:simulated intestinal fluid.


