FerruginineCAS# 73069-63-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 73069-63-3 | SDF | Download SDF |
| PubChem ID | 10012258 | Appearance | Powder |
| Formula | C10H15NO | M.Wt | 165.23 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
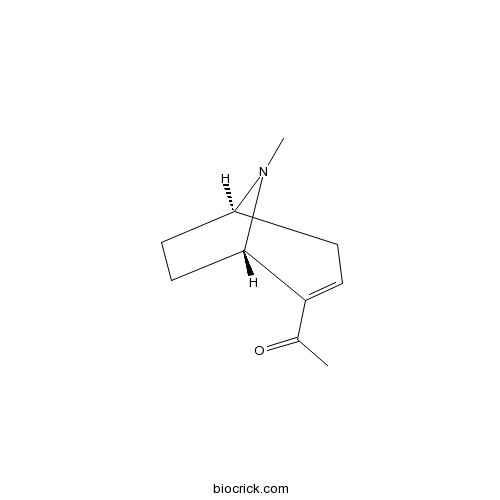
| Chemical Name | 1-[(1R,5S)-8-methyl-8-azabicyclo[3.2.1]oct-3-en-4-yl]ethanone | ||
| SMILES | CC(=O)C1=CCC2CCC1N2C | ||
| Standard InChIKey | KQIRSQYBYQBMIG-WPRPVWTQSA-N | ||
| Standard InChI | InChI=1S/C10H15NO/c1-7(12)9-5-3-8-4-6-10(9)11(8)2/h5,8,10H,3-4,6H2,1-2H3/t8-,10-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Targets | AChR |

Ferruginine Dilution Calculator

Ferruginine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.0522 mL | 30.2608 mL | 60.5217 mL | 121.0434 mL | 151.3042 mL |
| 5 mM | 1.2104 mL | 6.0522 mL | 12.1043 mL | 24.2087 mL | 30.2608 mL |
| 10 mM | 0.6052 mL | 3.0261 mL | 6.0522 mL | 12.1043 mL | 15.1304 mL |
| 50 mM | 0.121 mL | 0.6052 mL | 1.2104 mL | 2.4209 mL | 3.0261 mL |
| 100 mM | 0.0605 mL | 0.3026 mL | 0.6052 mL | 1.2104 mL | 1.513 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Arecoline
Catalog No.:BCN8537
CAS No.:73069-28-9
- Scutebarbatine D
Catalog No.:BCN8536
CAS No.:910099-76-2
- Praeruptorin D
Catalog No.:BCN4990
CAS No.:73069-28-0
- (+)-Praeruptorin A
Catalog No.:BCN4989
CAS No.:73069-27-9
- Praeruptorin A
Catalog No.:BCN4987
CAS No.:73069-25-7
- Atractylenolide II
Catalog No.:BCN1044
CAS No.:73069-14-4
- Atractylenolide I
Catalog No.:BCN1043
CAS No.:73069-13-3
- Epigomisin O
Catalog No.:BCN2862
CAS No.:73036-31-4
- Atractylenolide III
Catalog No.:BCN1045
CAS No.:73030-71-4
- CyPPA
Catalog No.:BCC7526
CAS No.:73029-73-9
- 15-Isopimarene-8,18-diol
Catalog No.:BCN4287
CAS No.:73002-86-5
- Lidocaine hydrochloride
Catalog No.:BCC9009
CAS No.:73-78-9
- Fenticonazole nitrate
Catalog No.:BCC8983
CAS No.:73151-29-8
- Effusol
Catalog No.:BCN2928
CAS No.:73166-28-6
- (d(CH2)51,Tyr(Me)2,Arg8)-Vasopressin
Catalog No.:BCC6011
CAS No.:73168-24-8
- Baptifoline
Catalog No.:BCN7988
CAS No.:732-50-3
- Xamoterol hemifumarate
Catalog No.:BCC6861
CAS No.:73210-73-8
- Chloranthalactone E
Catalog No.:BCN7466
CAS No.:73215-92-6
- Remoxipride hydrochloride
Catalog No.:BCC6844
CAS No.:73220-03-8
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
- Florfenicol
Catalog No.:BCC8984
CAS No.:73231-34-2
- Methylnaltrexone Bromide
Catalog No.:BCC1740
CAS No.:73232-52-7
- Moringin
Catalog No.:BCN7722
CAS No.:73255-40-0
- 5-O-Caffeoylshikimic acid
Catalog No.:BCN7929
CAS No.:73263-62-4
BF(3)-Induced rearrangement of aziridino cyclopropanes derived from 2-phenylsulfonyl 1,3-dienes. Application to the total synthesis of (+/-)-ferruginine.[Pubmed:11112563]
J Org Chem. 2000 Dec 15;65(25):8454-7.
Total synthesis of the alkaloid (+/-)-Ferruginine (1) has been developed via the 2-phenylsulfonyl 1,3-diene approach. BF(3)-induced rearrangement of the N-protected cyclohexane aziridino cyclopropane 8, derived from its corresponding epoxy cyclopropane, afforded the desired tropane alkaloid skeleton 9 in good yield. Michael addition of nitroethane (as an acyl anion equivalent) and transformation of the nitro group of the adduct 10 to a keto function gave 11. Elimination of benzenesulfinic acid and subsequent replacement of the tosyl group by a methyl group afforded the title compound 1.
Total synthesis of (-)-cocaine and (-)-ferruginine.[Pubmed:21391709]
J Org Chem. 2011 Apr 15;76(8):2694-700.
Total synthesis of tropane alkaloids (-)-cocaine and (-)-Ferruginine were accomplished in nine steps each and in 55% and 46% overall yields, respectively, starting from the known Betti base derivative (+)-(7aR,10R,12S)-10-(1H-benzotriazol-1-yl)-7a,8,9,10-tetrahydro-12-phenyl-12H-na phtho[1,2-e]pyrrolo[2,1-b][1,3]oxazine. In this novel route, RCM reaction and 1,3-dipolar cycloaddition were employed as key steps for the enantioselective construction of tropane skeleton and the regioselective introduction of 3-bromo-2-isoxazoline ring as masked cis-2,3-disubstituents. To obtain the desired precursor (2S,5R)-2-allyl-5-vinylpyrrolidine for RCM reaction, we developed a general and practical method for the preparation of enantiopure cis-2,5-disubstituted pyrrolidines bearing alkene- and/or alkyne-containing substituents. We also offered two highly efficient pathways for the conversion of the 3-bromo-2-isoxazoline ring into the desired cis-2,3-disubstituted groups in (-)-cocaine and (-)-Ferruginine.
Synthesis and evaluation of diazine containing bioisosteres of (-)-ferruginine as ligands for nicotinic acetylcholine receptors.[Pubmed:11557356]
Bioorg Med Chem. 2001 Oct;9(10):2683-91.
In this structure-affinity relationship (SAFIR) study, the bioisosteric potential of diazines in the field of Ferruginine-type nAChR ligands was investigated. Novel enantiopure analogues of (-)-Ferruginine (3) such as 6-8 were synthesized utilizing enantiomerically pure N-protected (+)-2-tropanone 9 from the 'chiral pool' as versatile chiral building block and a palladium-catalyzed Stille cross-coupling of the tributylstannyl diazines 12, 14 and 16 with the vinyl triflate 11 of (+)-2-tropanone 9. The structures of the novel diazine analogues 6-8 of (-)-Ferruginine (3) were assigned on the basis of spectral data, that of ligand 7 being additionally verified by X-ray crystallography. The bioisosteric replacement of the acetyl moiety as structural part of the lead compound 3 with the pyridazine, pyrimidine and pyrazine nucleus resulted in ligands with high to moderate affinity for the central alpha4beta2 and remarkably low affinity for the alpha7* nAChR subtypes. Among the compounds synthesized and tested, 7 was the most active one with K(i)=3.7 nM (alpha4beta2). Compared with the lead 3, this value represents a 30-fold improvement in the affinity for the alpha4beta2 subtype combined with a substantially improved selectivity ratio between the alpha4beta2 and alpha7* subtypes.


