trans-Aconitic acidCAS# 4023-65-8 |
- cis-Aconitic acid
Catalog No.:BCN0143
CAS No.:585-84-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4023-65-8 | SDF | Download SDF |
| PubChem ID | 444212 | Appearance | White powder |
| Formula | C6H6O6 | M.Wt | 174.1 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Freely soluble in ethanol and water; slightly soluble in diethyl ether | ||
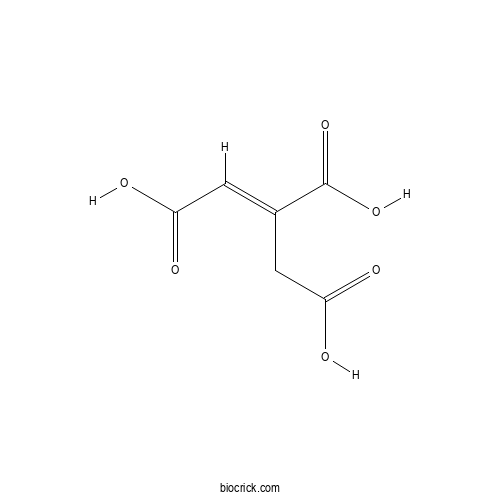
| Chemical Name | (E)-prop-1-ene-1,2,3-tricarboxylic acid | ||
| SMILES | C(C(=CC(=O)O)C(=O)O)C(=O)O | ||
| Standard InChIKey | GTZCVFVGUGFEME-HNQUOIGGSA-N | ||
| Standard InChI | InChI=1S/C6H6O6/c7-4(8)1-3(6(11)12)2-5(9)10/h1H,2H2,(H,7,8)(H,9,10)(H,11,12)/b3-1+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Trans-aconitic acid inhibits the growth and photosynthesis of Glycine max.Trans-aconitic acid has antileishmanial activity ,the diesters of TAA as potential useful derivatives for the management of rheumatoid arthritis and other inflammatory diseases. | |||||

trans-Aconitic acid Dilution Calculator

trans-Aconitic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.7438 mL | 28.7191 mL | 57.4383 mL | 114.8765 mL | 143.5956 mL |
| 5 mM | 1.1488 mL | 5.7438 mL | 11.4877 mL | 22.9753 mL | 28.7191 mL |
| 10 mM | 0.5744 mL | 2.8719 mL | 5.7438 mL | 11.4877 mL | 14.3596 mL |
| 50 mM | 0.1149 mL | 0.5744 mL | 1.1488 mL | 2.2975 mL | 2.8719 mL |
| 100 mM | 0.0574 mL | 0.2872 mL | 0.5744 mL | 1.1488 mL | 1.436 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Quercetin-3'-glucoside
Catalog No.:BCN9987
CAS No.:19254-30-9
- 1,3-Diphenyl-2-propen-1-one
Catalog No.:BCN9986
CAS No.:94-41-7
- 4'-Methoxyflavanone
Catalog No.:BCN9985
CAS No.:97005-76-0
- Farnesol
Catalog No.:BCN9984
CAS No.:4602-84-0
- (R,S)-Equol
Catalog No.:BCN9983
CAS No.:66036-38-2
- 4',6,7-Trimethoxyisoflavone
Catalog No.:BCN9982
CAS No.:798-61-8
- trans-Beta-Apo-8'-carotenal
Catalog No.:BCN9981
CAS No.:1107-26-2
- (-)-Verbenone
Catalog No.:BCN9980
CAS No.:1196-01-6
- Harmol
Catalog No.:BCN9979
CAS No.:487-03-6
- Henricine
Catalog No.:BCN9978
CAS No.:107783-46-0
- omega-Benzoyl oxyphloracetophenone
Catalog No.:BCN9977
CAS No.:65982-77-6
- AT101
Catalog No.:BCN9976
CAS No.:866541-93-7
- 3-Octyl alcohol
Catalog No.:BCN9989
CAS No.:589-98-0
- Phloroglucinol aldehyde triethylether
Catalog No.:BCN9990
CAS No.:59652-88-9
- Daclatasvir
Catalog No.:BCN9991
CAS No.:1009119-64-5
- Geranylacetate
Catalog No.:BCN9992
CAS No.:105-87-3
- Cytochalasin C
Catalog No.:BCN9993
CAS No.:22144-76-9
- 4-Hydroxyquinoline
Catalog No.:BCN9994
CAS No.:611-36-9
- 2',3,5,7-Tetrahydroxyflavone
Catalog No.:BCN9995
CAS No.:480-15-9
- 3,4-Dimethoxychalcone
Catalog No.:BCN9996
CAS No.:5416-71-7
- Oxyacanthine hydrochloride
Catalog No.:BCN9997
CAS No.:15352-74-6
- 7-Methoxyflavone
Catalog No.:BCN9998
CAS No.:22395-22-8
- trans-5-Hydroxyferulic acid
Catalog No.:BCN9999
CAS No.:110642-42-7
- 8-p-Coumaroylharpagide
Catalog No.:BCN0152
CAS No.:87686-74-6
Plasma and urine metabolomic analyses in aortic valve stenosis reveal shared and biofluid-specific changes in metabolite levels.[Pubmed:33237940]
PLoS One. 2020 Nov 25;15(11):e0242019.
Aortic valve stenosis (AVS) is a prevalent condition among the elderly population that eventually requires aortic valve replacement. The lack of reliable biomarkers for AVS poses a challenge for its early diagnosis and the application of preventive measures. Untargeted gas chromatography mass spectrometry (GC-MS) metabolomics was applied in 46 AVS cases and 46 controls to identify plasma and urine metabolites underlying AVS risk. Multivariate data analyses were performed on pre-processed data (e.g. spectral peak alignment), in order to detect changes in metabolite levels in AVS patients and to evaluate their performance in group separation and sensitivity of AVS prediction, followed by regression analyses to test for their association with AVS. Through untargeted analysis of 190 urine and 130 plasma features that could be detected and quantified in the GC-MS spectra, we identified contrasting levels of 22 urine and 21 plasma features between AVS patients and control subjects. Following metabolite assignment, we observed significant changes in the concentration of known metabolites in urine (n = 14) and plasma (n = 15) that distinguish the metabolomic profiles of AVS patients from healthy controls. Associations with AVS were replicated in both plasma and urine for about half of these metabolites. Among these, 2-Oxovaleric acid, elaidic acid, myristic acid, palmitic acid, estrone, myo-inositol showed contrasting trends of regulation in the two biofluids. Only trans-Aconitic acid and 2,4-Di-tert-butylphenol showed consistent patterns of regulation in both plasma and urine. These results illustrate the power of metabolomics in identifying potential disease-associated biomarkers and provide a foundation for further studies towards early diagnostic applications in severe heart conditions that may prevent surgery in the elderly.
Development of Antioxidant-Loaded Nanoliposomes Employing Lecithins with Different Purity Grades.[Pubmed:33207762]
Molecules. 2020 Nov 16;25(22). pii: molecules25225344.
This work focused on comparing the ability of lecithins with two purity grades regarding their performance in the development of nanoliposomes, as well as their ability to contain and release polar (trans-Aconitic acid) and non-polar (quercetin) antioxidant compounds. First, the chemical characterization of both lecithins was carried out through infrared spectroscopy (FTIR), electrospray ionization mass spectrometry (ESI/MS), and modulated differential scanning calorimetry (mDSC). Second, nanoliposomes were prepared by the ethanol injection method and characterized by means of particle size, polydispersity, and zeta potential measurements. Third, the encapsulation efficiency and in vitro release profiles of antioxidants were evaluated. Finally, the antioxidant effect of quercetin and trans aconitic acid in the presence and absence of nanoliposomes was assessed through the oxygen radical absorbance capacity (ORAC) assay. The results showed that, although there are differences in the chemical composition between the two lecithins, these allow the development of nanoliposomes with very similar physicochemical features. Likewise, nanoliposomes elaborated with low purity grade lecithins favored the encapsulation and release of trans-Aconitic acid (TAA), while the nanoliposomes made with high purity lecithins favored the encapsulation of quercetin (QCT) and modified its release. Regarding the antioxidant effect, the vehiculization of TAA and QCT in nanoliposomes led to an increase in the antioxidant capability, where QCT showed a sustained effect over time and TAA exhibited a rapidly decaying effect. Likewise, liposomal systems were also found to have a slight antioxidant effect.
High quantum yield photoluminescent N-doped carbon dots for switch sensing and imaging.[Pubmed:33167278]
Talanta. 2021 Jan 15;222:121663.
Stable blue fluorescent nitrogen doped carbon dots (N-CDs) with a very high quantum yield up to 81% has been reported for the first time. Novel N-CDs were synthesized through an efficient and rapid one-step hydrothermal synthesis process from diethylenetriamine as nitrogen source and a novel carbon source trans-Aconitic acid. The nanosized particles of N-CDs were in the range of 2-8 nm and uniformly distributed in molecular level. The N-CDs showed high selectivity toward Fe(3+) with low detection limit of 10.42 nmol L(-1) (with corresponding linear range of 2-50 mumol L(-1)) enabling them for ion detection application and also exhibited high fluorescence stability in extreme pH conditions. Novel N-CDs also presented a green emission shift under acidic condition (pH~2) which makes them a potential sensing probe for security papers, food packaging and bio-medical detection sensors. A security paper sensor device has been fabricated and its operation function has been validated by making real time detection of color. The novel and facile to manufacture carbon dots has potential applications ranging from biological nano-sensors for security document to color-switch sensing and bio-imaging.
Constitutive production of aconitate isomerase by Pseudomonas sp. WU-0701 in relation to trans-aconitic acid assimilation.[Pubmed:32994133]
J Biosci Bioeng. 2020 Sep 26. pii: S1389-1723(20)30335-2.
Aconitic acid, an unsaturated tricarboxylic acid, is used in the chemical industry as raw materials for organic synthesis, especially as a specific substrate for a flavoring agent. trans-Aconitic acid (tAA) is a trans-isomer of cis-aconitic acid and detected in some plants and bacteria. However, biosynthetic route and metabolism of tAA in relation to assimilation have been unknown. Aconitate isomerase (AI; EC 5.3.3.7) catalyzes the reversible isomerization between cis-aconitic acid and tAA. Pseudomonas sp. WU-0701 was isolated as a bacterium assimilating tAA as sole carbon source, and characterization and gene identification of AI were already reported. Here, we describe that Pseudomonas sp. WU-0701 exhibited growth in each synthetic medium containing glucose, citric acid, isocitric acid, or tAA as sole carbon source. AI was intracellularly detected all the time during the cultivation of the strain WU-0701 cells, irrespective of the carbon sources; AI activity was detected even in the glucose-grown cells. Through the subcellular fractionation experiments, AI was detected in the periplasmic fraction. This is the first report indicating that a bacterium belonging to the genus Pseudomonas is constitutive for the AI production.
Microencapsulation of Fluticasone Propionate and Salmeterol Xinafoate in Modified Chitosan Microparticles for Release Optimization.[Pubmed:32859128]
Molecules. 2020 Aug 26;25(17). pii: molecules25173888.
Chitosan (CS) is a natural polysaccharide, widely studied in the past due to its unique properties such as biocompatibility, biodegradability and non-toxicity. Chemical modification of CS is an effective pathway to prepare new matrices with additional functional groups and improved properties, such as increment of hydrophilicity and swelling rate, for drug delivery purposes. In the present study, four derivatives of CS with trans-Aconitic acid (t-Acon), succinic anhydride (Succ), 2-hydroxyethyl acrylate (2-HEA) and acrylic acid (AA) were prepared, and their successful grafting was confirmed by FTIR and (1)H-NMR spectroscopies. Neat chitosan and its grafted derivatives were fabricated for the encapsulation of fluticasone propionate (FLU) and salmeterol xinafoate (SX) drugs, used for chronic obstructive pulmonary disease (COPD), via the ionotropic gelation technique. Scanning electron microscopy (SEM) micrographs demonstrated that round-shaped microparticles (MPs) were effectively prepared with average sizes ranging between 0.4 and 2.2 mum, as were measured by dynamic light scattering (DLS), while zeta potential verified in all cases their positive charged surface. FTIR spectroscopy showed that some interactions take place between the drugs and the polymeric matrices, while X-ray diffraction (XRD) patterns exhibited that both drugs were encapsulated in MPs' interior with a lower degree of crystallinity than the neat drugs. In vitro release studies of FLU and SX exposed a great amelioration in the drugs' dissolution profile from all modified CS's MPs, in comparison to those of neat drugs. The latter fact is attributed to the reduction in crystallinity of the active substances in the MPs' interior.
Effect of coexisting manganese ion on the formation of haloacetic acids during chlorination.[Pubmed:32814132]
Chemosphere. 2021 Jan;263:127862.
Haloacetic acids (HAAs) are a group of disinfection by-products formed by the reaction of dissolved organic matter (DOM) in source water and disinfectants in the drinking water treatment process. The formation of HAAs is known to be affected by several factors (e.g., pH, temperature, concentration, and DOM components in source water). However, the effects of coexisting substances, such as metal ions, on HAA formation are not well understood. In this study, HAA formation potentials (FPs) of model compounds of DOM and environmental waters in the presence or absence of manganese ion upon chlorination were compared. The results of experiments with model compounds of DOM showed that manganese ion promoted the formation of HAA from citric acid, trans-Aconitic acid, and cis-aconitic acid. Even for a manganese concentration of less than 50 mug/L, which is the standard value of manganese in drinking water in the USA, EU, and Japan, manganese had great influence on the dichloroacetic acid FPs of these compounds. However, the manganese ion did not enhance the HAAFPs of the environmental waters tested. Nevertheless, manganese may have an effect on HAAFPs of environmental waters collected at the occurrence of an unusual growth of microorganisms, such as algal bloom.
Electroactivity of polyphenols in sweet sorghum (Sorghum bicolor (L.) Moench) cultivars.[Pubmed:32663216]
PLoS One. 2020 Jul 14;15(7):e0234509.
Polyphenols and other potential health-promoting components of sorghum (Sorghum bicolor (L.) Moench) drove its recent growth in the U.S. consumer food industry. Linear sweep (cyclic voltammetry, CV) and differential (cyclic differential pulse) voltammetry methods were developed to detect target polyphenols and amino acids in sweet sorghum juice without interference from the dominant secondary (trans-Aconitic acid) and primary (sucrose) metabolites. Of 24 cultivars investigated, No.5 Gambela showed the highest electron-donating capacity, as indicated by the highest peak area, height, and peak anodic potential. Pearson's correlation analysis indicated the contribution of polyphenols (rather than amino acids) on CV voltammograms of juice samples. The Eh-pH values of 173 sweet sorghum juice samples collected in 2017 aligned with quercetin model polyphenol. Accumulation of quercetin-like polyphenols in No.5 Gambela could offer antioxidant-rich juice for conversion to edible syrup as well as an increased tolerance against a recently emerged pest, sugarcane aphid [(Melanaphis sacchari (Zehntner)].
Accumulation of Carboxylate and Aromatic Fluorophores by a Pest-Resistant Sweet Sorghum [Sorghum bicolor (L.) Moench] Genotype.[Pubmed:31858036]
ACS Omega. 2019 Nov 27;4(24):20519-20529.
The sugary juice from sweet sorghum [Sorghum bicolor (L.) Moench] stalks can be used to produce edible syrup, biofuels, or bio-based chemical feedstock. The current cultivars are highly susceptible to damage from sugarcane aphids [Melanaphis sacchari (Zehntner)], but development of new cultivars is hindered by a lack of rapid analytical methods to screen for juice quality traits. The mechanism of aphid resistance/tolerance is also largely unknown, though the importance of defense phytochemicals has been suggested. The purpose of this study was to develop low-cost methods sensitive to fluorescent fingerprints in sweet sorghum juice, which is a complex mixture of saccharides, carboxylates, polyphenols, and metal ions. Of primary juice components, tryptophan and trans-Aconitic acid were the highest intensity contributors to the overall fluorescence and UV/visible absorbance, respectively, while tyrosine and polyphenols contributed to a less extent. In a test of 24 sweet sorghum cultivars, tryptophan and tyrosine contents were the highest in the aphid-susceptible hybrid N109A x Chinese, while sucrose, trans-Aconitic acid, and polyphenols were the highest in the resistant line No. 5 Gambela. This suggests that the accumulation of carboxylate (trans-Aconitic acid) and polyphenolic secondary products in No. 5 Gambela may contribute to its aphid resistance, thus allowing it to maintain sucrose production. Rapid detection of these chemical signatures could be used to prescreen the breeding material for potential resistance and juice quality traits, without analytical separation required for metabolomics.
Chitosan Grafted Adsorbents for Diclofenac Pharmaceutical Compound Removal from Single-Component Aqueous Solutions and Mixtures.[Pubmed:30960481]
Polymers (Basel). 2019 Mar 14;11(3). pii: polym11030497.
The main purpose of this study was to investigate the synthesis of some cross-linked carboxyl-grafted chitosan derivatives to be used as selective adsorbents for diclofenac (DCF) pharmaceutical compounds from aqueous mixtures. Four different materials were synthesized using succinic anhydride (CsSUC), maleic anhydride (CsMAL), itaconic acid (CsITA), and trans-Aconitic acid (CsTACON) as grafting agents. After synthesis, scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FTIR), and X-ray diffraction (XRD) were performed before and after DCF adsorption. In addition, a complete adsorption evaluation was carried out for all materials studying some important parameters. The optimum pH was 4; the amino groups of DCF can be protonated at pH = 4 ((-)NH(+)), so this groups can easily attract the clear negatively carboxyl moieties ((-)COO(-)) of the chitosan adsorbents. The Qm for CsTACON was higher than those of the other materials, at all temperatures studied. By altering the temperature from 25 to 35 degrees C, an increase (16%) of Qm (from 84.56 to 98.34 mg g(-1)) was noted, while similar behavior was revealed after a further increase of temperature from 35 to 45 degrees C, improving by 5% (from 98.34 to 102.75 mg g(-1)). All isotherms were fitted to Langmuir, Freundlich, and Langmuir-Freundlich (L-F) models). In addition, a kinetic model was proposed taking into account not only the interactions but also the diffusivity of the molecule (DCF) into the polymeric network. The behavior of the prepared chitosan materials in simultaneously removing other compounds (synergetic or antagonistic) was also evaluated by experiments performed in mixtures. DCF presented the highest removal from the mixture in the order: CsTACON (92.8%) > CsITA (89.5%) > CsSUC (80.9%) > CsMAL (66.2%) compared to other pharmaceutical compounds (salicylic acid, ibuprofen and ketoprofen). Desorption was achieved by using different eluants (either water or organic). The highest desorption ability was found for acetone (100% for CsTACON, CsSUC, CsMAL and 77% for CsITA) for all materials.
Rapid Data Analytics to Relate Sugarcane Aphid [(Melanaphis sacchari (Zehntner)] Population and Damage on Sorghum (Sorghum bicolor (L.) Moench).[Pubmed:30674945]
Sci Rep. 2019 Jan 23;9(1):370.
Sugarcane aphid [(Melanaphis sacchari (Zehntner)] emerged in the United States in 2013 as a new pest infesting sorghum (Sorghum bicolor (L.) Moench). Aphid population and plant damage are assessed by field scouting with mean comparison tests or repeated regression analysis. Because of inherently large replication errors from the field and interactions between treatments, new data analytics are needed to rapidly visualize the pest emergence trend and its impact on plant damage. This study utilized variable importance in the projection (VIP) and regression vector statistics of partial least squares (PLS) modeling to deduce directional relationships between aphid population and leaf damage from biweekly field monitoring (independent variable) and chemical composition (dependent variable) of 24 sweet sorghum cultivars. Regardless of environment, aphid population increase preceded the maximum damage rating. Greater damage rating at earlier growth stage in 2015 than 2016 led to an overall higher damage rating in 2015 than 2016. This trend in damage coincided with higher concentrations of trans-Aconitic acid and polyphenolic secondary products in stem juice in 2016 than 2015, at the expense of primary sugar production. Developed rapid data analytics could be extended to link phenotypes to perturbation parameters (e.g., cultivar and growth stage), enabling integrated pest management.
Trans-aconitic acid inhibits the growth and photosynthesis of Glycine max.[Pubmed:30292981]
Plant Physiol Biochem. 2018 Nov;132:490-496.
Grasses producing trans-Aconitic acid, a geometric isomer of cis-aconitic acid, are often used in Glycine max rotation systems. However, the effects of trans-Aconitic acid on Glycine max are unknown. We conducted a hydroponic experiment to evaluate the effects of 2.5-10mM trans-Aconitic acid on Glycine max growth and photosynthesis. The results revealed that the enhanced H2O2 production in the roots increased the membrane permeability and reduced the water uptake. These effects culminated with a reduced stomatal conductance (gs), which seems to be the main cause for a decreased photosynthetic rate (A). Due to low gs, the limited CO2 assimilation may have overexcited the photosystems, as indicated by the high production of H2O2 in leaves. After 96h of incubation, and due to H2O2-induced damage to photosystems, a probable non-stomatal limitation for photosynthesis contributed to reducing A. This is corroborated by the significant decrease in the quantum yield of electron flow through photosystem II in vivo (PhiPSII) and the chlorophyll content. Taken together, the damage to the root system and photosynthetic apparatus caused by trans-Aconitic acid significantly reduced the Glycine max plant growth.
Encapsulation of trans-aconitic acid in mucoadhesive microspheres prolongs the anti-inflammatory effect in LPS-induced acute arthritis.[Pubmed:29627623]
Eur J Pharm Sci. 2018 Jul 1;119:112-120.
trans-Aconitic acid (TAA) is the main constituent of the leaves from the medicinal plant Echinodorus grandiflorus, used to treat different inflammatory diseases. TAA induces a potent but short-lasting biological response, credited to its high polarity and unfavorable pharmacokinetics. Here we developed, characterized and evaluated the anti-inflammatory activity of mucoadhesive microspheres loaded with TAA. Seven batches of mucoadhesive microspheres were prepared by the emulsification/solvent evaporation method, employing different proportions of TAA and Carbopol 934 or/and hydroxypropylmethylcellulose. All batches were characterized for their particle medium size, polydispersity index and entrapment percentage. The batch coded F3c showed highest entrapment percentage and was characterized by infrared spectroscopy (ATR-FTIR), scanning electron microscopy (SEM), differential scanning calorimetry (DSC), thermogravimetric analyses (TGA) and zeta potential. The anti-inflammatory activity of F3c was assessed in a model of acute arthritis induced by injection of LPS in the knee joint of Swiss mice. The granulometric analyses indicated heterogeneous size distribution for F3c. SEM characterization indicated microspheres with slightly irregular shape and rough surface. Results from ATR-FTIR and thermal analyses (DSC and TGA) pointed out absence of incompatibility between the components of the formulation; thermal events related to the constituents were isolated and randomly located, suggesting amorphous distribution of TAA in the formulation matrix. The zeta potential of the formulations varied from -30 to -34mV, which may contribute to good stability. When given orally to mice, F3c induced a prolonged anti-inflammatory response by reducing total cell count and neutrophilic accumulation in the joint cavity even when given 48 and 36h before the stimulus, respectively, in comparison to free TAA (up to 24 and 6h, respectively). Therefore, the encapsulation of TAA in mucoadhesive microspheres provided its sustained release, indicating that this drug delivery system is a potential agent to treat inflammatory diseases by regulating cell influx.
Esterification of trans-aconitic acid improves its anti-inflammatory activity in LPS-induced acute arthritis.[Pubmed:29329035]
Biomed Pharmacother. 2018 Mar;99:87-95.
trans-Aconitic acid (TAA) is an abundant constituent in the leaves of Echinodorus grandiflorus, a medicinal plant used to treat rheumatoid arthritis in Brazil. Esterification was explored as a strategy to increase lipophilicity and biopharmaceutical properties of TAA, a highly polar tricarboxylic acid. We herein report the synthesis of TAA esters via Fischer esterification with ethanol, n-butanol and n-octanol. The reaction kinetics was investigated to produce mono-, di- and tri- derivatives. Mono- and diesters of TAA were obtained as a mixture of positional isomers, whereas the triesters were recovered as pure compounds. The obtained esters were screened in a model of acute arthritis induced by the injection of LPS in the knee joint of Swiss mice. The diesters were the most active compounds, regardless of the alcohol employed in the reaction, whereas bioactivity of the derivatives improved by increasing the length of the aliphatic chain of the alcohol employed in esterification. In general, the esters showed higher potency than TAA. When administered orally to mice at doses of 0.017-172.3mumol/Kg, the diethyl, di-n-butyl and di-n-octyl esters of TAA reduced the cellular infiltration into the knee joint, especially of neutrophils. The study identified diesters of TAA as potential useful derivatives for the management of rheumatoid arthritis and other inflammatory diseases.


