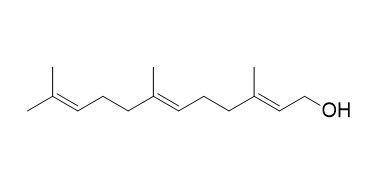
FarnesolCAS# 4602-84-0 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 4602-84-0 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Oil |
| Formula | C15H26O | M.Wt | 222.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Farnesol has anti-bacterial activity, it inhibits PQS production in Pseudomonas aeruginosa, it promotes epithelial cell defense against Candida albicans through Toll-like receptor 2 expression, interleukin-6 and human β-defensin 2 production.Farnesol stimulates differentiation in Epidermal Keratinocytes via PPARα. It stimulates MCF-7 breast cancer cell growth through farnesoid-X-receptor-mediated estrogen receptor activation. | |||||

Farnesol Dilution Calculator

Farnesol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4984 mL | 22.4921 mL | 44.9843 mL | 89.9685 mL | 112.4606 mL |
| 5 mM | 0.8997 mL | 4.4984 mL | 8.9969 mL | 17.9937 mL | 22.4921 mL |
| 10 mM | 0.4498 mL | 2.2492 mL | 4.4984 mL | 8.9969 mL | 11.2461 mL |
| 50 mM | 0.09 mL | 0.4498 mL | 0.8997 mL | 1.7994 mL | 2.2492 mL |
| 100 mM | 0.045 mL | 0.2249 mL | 0.4498 mL | 0.8997 mL | 1.1246 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (R,S)-Equol
Catalog No.:BCN9983
CAS No.:66036-38-2
- 4',6,7-Trimethoxyisoflavone
Catalog No.:BCN9982
CAS No.:798-61-8
- trans-Beta-Apo-8'-carotenal
Catalog No.:BCN9981
CAS No.:1107-26-2
- (-)-Verbenone
Catalog No.:BCN9980
CAS No.:1196-01-6
- Harmol
Catalog No.:BCN9979
CAS No.:487-03-6
- Henricine
Catalog No.:BCN9978
CAS No.:107783-46-0
- omega-Benzoyl oxyphloracetophenone
Catalog No.:BCN9977
CAS No.:65982-77-6
- AT101
Catalog No.:BCN9976
CAS No.:866541-93-7
- 9-Methyl-9-azabicyclo[3.3.1]nonan-3-one
Catalog No.:BCN9975
CAS No.:552-70-5
- Platycogenin A
Catalog No.:BCN9974
CAS No.:1459719-53-9
- Peltatoside
Catalog No.:BCN9973
CAS No.:23284-18-6
- Terpinyl acetate
Catalog No.:BCN9972
CAS No.:80-26-2
- 4'-Methoxyflavanone
Catalog No.:BCN9985
CAS No.:97005-76-0
- 1,3-Diphenyl-2-propen-1-one
Catalog No.:BCN9986
CAS No.:94-41-7
- Quercetin-3'-glucoside
Catalog No.:BCN9987
CAS No.:19254-30-9
- trans-Aconitic acid
Catalog No.:BCN9988
CAS No.:4023-65-8
- 3-Octyl alcohol
Catalog No.:BCN9989
CAS No.:589-98-0
- Phloroglucinol aldehyde triethylether
Catalog No.:BCN9990
CAS No.:59652-88-9
- Daclatasvir
Catalog No.:BCN9991
CAS No.:1009119-64-5
- Geranylacetate
Catalog No.:BCN9992
CAS No.:105-87-3
- Cytochalasin C
Catalog No.:BCN9993
CAS No.:22144-76-9
- 4-Hydroxyquinoline
Catalog No.:BCN9994
CAS No.:611-36-9
- 2',3,5,7-Tetrahydroxyflavone
Catalog No.:BCN9995
CAS No.:480-15-9
- 3,4-Dimethoxychalcone
Catalog No.:BCN9996
CAS No.:5416-71-7
An automated workflow to screen alkene reductases using high-throughput thin layer chromatography.[Pubmed:33292503]
Biotechnol Biofuels. 2020 Nov 9;13(1):184.
BACKGROUND: Synthetic biology efforts often require high-throughput screening tools for enzyme engineering campaigns. While innovations in chromatographic and mass spectrometry-based techniques provide relevant structural information associated with enzyme activity, these approaches can require cost-intensive instrumentation and technical expertise not broadly available. Moreover, complex workflows and analysis time can significantly impact throughput. To this end, we develop an automated, 96-well screening platform based on thin layer chromatography (TLC) and use it to monitor in vitro activity of a geranylgeranyl reductase isolated from Sulfolobus acidocaldarius (SaGGR). RESULTS: Unreduced SaGGR products are oxidized to their corresponding epoxide and applied to thin layer silica plates by acoustic printing. These derivatives are chromatographically separated based on the extent of epoxidation and are covalently ligated to a chromophore, allowing detection of enzyme variants with unique product distributions or enhanced reductase activity. Herein, we employ this workflow to examine Farnesol reduction using a codon-saturation mutagenesis library at the Leu377 site of SaGGR. We show this TLC-based screen can distinguish between fourfold differences in enzyme activity for select mutants and validated those results by GC-MS. CONCLUSIONS: With appropriate quantitation methods, this workflow can be used to screen polyprenyl reductase activity and can be readily adapted to analyze broader catalyst libraries whose products are amenable to TLC analysis.
Antibiofilm and antifungal activities of medium-chain fatty acids against Candida albicans via mimicking of the quorum-sensing molecule farnesol.[Pubmed:33252828]
Microb Biotechnol. 2020 Nov 30.
Candida biofilms are tolerant to conventional antifungal therapeutics and the host immune system. The transition of yeast cells to hyphae is considered a key step in C. albicans biofilm development, and this transition is inhibited by the quorum-sensing molecule Farnesol. We hypothesized that fatty acids mimicking Farnesol might influence hyphal and biofilm formation by C. albicans. Among 31 saturated and unsaturated fatty acids, six medium-chain saturated fatty acids, that is, heptanoic acid, octanoic acid, nonanoic acid, decanoic acid, undecanoic acid and lauric acid, effectively inhibited C. albicans biofilm formation by more than 75% at 2 microg ml(-1) with MICs in the range 100-200 microg ml(-1) . These six fatty acids at 2 microg ml(-1) and Farnesol at 100 microg ml(-1) inhibited hyphal growth and cell aggregation. The addition of fatty acids to C. albicans cultures decreased the productions of Farnesol and sterols. Furthermore, down-regulation of several hyphal and biofilm-related genes caused by heptanoic or nonanoic acid closely resembled the changes caused by Farnesol. In addition, nonanoic acid, the most effective compound diminished C. albicans virulence in a Caenorhabditis elegans model. Our results suggest that medium-chain fatty acids inhibit more effectively hyphal growth and biofilm formation than Farnesol.
Farnesol induces mitochondrial/peroxisomal biogenesis and thermogenesis by enhancing the AMPK signaling pathway in vivo and in vitro.[Pubmed:33246168]
Pharmacol Res. 2020 Nov 24;163:105312.
Thermogenic activation of brown adipose tissue has been considered as an obesity treatment strategy that consumes energy. In this study, we investigated whether Farnesol in vivoandin vitro models induces thermogenesis and affect the activation of the mitochondria and peroxisomes, which are key organelles in activated brown adipocytes. Farnesol induced the expression of thermogenic factors such as uncoupling protein 1 (UCP1), peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1alpha), and PR domain zinc-finger protein 16 (PRDM16) together with the phosphorylation of AMP-activated protein kinase alpha (AMPKalpha) in brown adipose tissue and primary cultured brown adipocytes. Farnesol promoted lipolytic enzymes: hormone sensitive lipase (HSL) and adipose triglyceride lipase (ATGL). We confirmed that these inductions of lipolysis by Farnesol were the underlying causes of beta-oxidation activation. Farnesol also increased the expression of oxidative phosphorylation (OXPHOS) complexes and the oxygen consumption rate (OCR) and the expansion of peroxisomes. Moreover, we proved that the thermogenic activity of Farnesol was dependent on AMPKalpha activation using Compound C inhibitor or siRNA-AMPKalpha knockdown. These results suggest that Farnesol may be a potential agent for the treatment of obesity by inducing energy consumption through heat generation.
Search for Alternative Control Strategies of Drosophila suzukii (Diptera: Drosophilidae): Laboratory Assays Using Volatile Natural Plant Compounds.[Pubmed:33217940]
Insects. 2020 Nov 18;11(11). pii: insects11110811.
Drosophila suzukii (Diptera: Drosophilidae), is native to southeastern Asia and invaded Europe during the past decade. It causes serious economic damage in cherries and soft fruits. Control strategies rely on few insecticides with varying success. Due to environmental concern, the use of synthetic chemicals is restricted. Therefore, research effort is put into the quest for alternative substances applicable in chemical pest control. In laboratory assays, we tested 17 volatile plant compounds from different chemical classes for their contact toxicity, feeding modification, and oviposition repellency. Toxicity through contact with treated surfaces was evaluated after 1 h, 4 h, and 24 h; effects on food uptake were observed with capillary feeding (CAFE)-tests and oviposition trials compared egg numbers laid in raspberry medium with or without treated filter paper. Cinnamon oil and its components had the highest contact toxicity with an LC90 = 2-3%, whereas lemongrass oil, its main components, and Farnesol were less toxic (LC90 = 7-9%), and geraniol was the least toxic. In CAFE tests, feeding stimulation was observed through 0.1% and 1% solutions of citronellol, lemongrass oil and Farnesol. Cinnamon oil, cinnamaldhyde, and ethyl cinnamate were not consumed at a concentration of 1%. In the presence of citral, eugenol, and lemongrass oil, oviposition was reduced, and in the presence of limonene, no eggs were deposited. The natural products found most efficient in either bioassay will be further tested under field conditions.
Two Undervalued Functions of the Golgi Apparatus: Removal of Excess Ca(2+) and Biosynthesis of Farnesol-Like Sesquiterpenoids, Possibly as Ca(2+)-Pump Agonists and Membrane "Fluidizers-Plasticizers".[Pubmed:33178030]
Front Physiol. 2020 Oct 15;11:542879.
The extensive literature dealing with the Golgi system emphasizes its role in protein secretion and modification, usually without specifying from which evolutionary ancient cell physiological necessity such secretion originated. Neither does it specify which functional requirements the secreted proteins must meet. From a reinterpretation of some classical and recent data gained mainly, but not exclusively, from (insect) endocrinology, the view emerged that the likely primordial function of the rough endoplasmic reticulum (RER)-Golgi complex in all eukaryotes was not the secretion of any type of protein but the removal of toxic excess Ca(2+) from the cytoplasm. Such activity requires the concurrent secretion of large amounts of Ca(2+)-carrying/transporting proteins acting as a micro-conveyor belt system inside the RER-Golgi. Thus, (fitness increasing) protein secretion is subordinate to Ca(2+) removal. Milk with its high content of protein and Ca(2+) (60-90 mM vs. 100 nM in unstimulated mammary gland cells) is an extreme example. The sarco(endo)plasmatic reticulum Ca(2+)-ATPases (SERCAs) and SPCA1a Ca(2+)/Mn(2+) transport ATPases are major players in Ca(2+) removal through the Golgi. Both are blocked by the sesquiterpenoid thapsigargin. This strengthens the hypothesis (2014) that endogenous Farnesol-like sesquiterpenoids (FLSs) may act as the long sought for but still unidentified agonist(s) for Ca(2+)-pumps in both the ER and Golgi. A second putative function also emerges. The fusion of both the incoming and outgoing transport vesicles, respectively, at the cis- and trans- side of Golgi stacks, with the membrane system requiring high flexibility and fast self-closing of the involved membranes. These properties may-possibly partially-be controlled by endogenous hydrophobic membrane "fluidizers" for which FLSs are prime candidates. A recent reexamination of unexplained classical data suggests that they are likely synthesized by the Golgi itself. This game-changing hypothesis is endorsed by several arguments and data, some of which date from 1964, that the insect corpus allatum (CA), which is the major production site of Farnesol-esters, has active Golgi systems. Thus, in addition to secreting FLS, in particular juvenile hormone(s), it also secretes a protein(s) or peptide(s) with thus far unknown function. This paper suggests answers to various open questions in cell physiology and general endocrinology.
Longer Ubiquinone Side Chains Contribute to Enhanced Farnesol Resistance in Yeasts.[Pubmed:33114039]
Microorganisms. 2020 Oct 23;8(11). pii: microorganisms8111641.
Ubiquinones (UQ) are intrinsic lipid components of many membranes. Besides their role in electron-transfer reactions there is evidence for them acting as free radical scavengers, yet their other roles in biological systems have received little study. The dimorphic fungal pathogen Candida albicans secretes Farnesol as both a virulence factor and a quorum-sensing molecule. Thus, we were intrigued by the presence of UQ9 isoprenologue in Farnesol-producing Candida species while other members of this genera harbor UQ7 as their major electron carrier. We examined the effect of UQ side chain length in Saccharomyces cerevisiae and C. albicans with a view towards identifying the mechanisms by which C. albicans protects itself from the high levels of Farnesol it secretes, levels that are toxic to many other fungi including S. cerevisiae. In this study, we identify UQ9 as the major UQ isoprenoid in C. albicans, regardless of growth conditions or cell morphology. A S. cerevisiae model yeast engineered to make UQ9 instead of UQ6 was 4-5 times more resistant to exogenous Farnesol than the parent yeast and this resistance was accompanied by greatly reduced reactive oxygen species (ROS) production. The resistance provided by UQ9 is specific for Farnesol in that it does not increase resistance to high salt (1M NaCl) or other oxidants (5 mM H2O2 or 1 mM menadione). Additionally, the protection provided by UQ9 appears to be structural rather than transcriptional; UQ9 does not alter key transcriptional responses to Farnesol stress. Here, we propose a model in which the longer UQ side chains are more firmly embedded in the mitochondrial membrane making them harder to pry out, so that in the presence of Farnesol they remain functional without producing excess ROS. C. albicans and Candida dubliniensis evolved to use UQ9 rather than UQ7 as in other Candida species or UQ6 as in S. cerevisiae. This adaptive mechanism highlights the significance of UQ side chains in Farnesol production and resistance quite apart from being an electron carrier in the respiratory chain.
Exogenous fungal quorum sensing molecules inhibit planktonic cell growth and modulate filamentation and biofilm formation in the Sporothrix schenckii complex.[Pubmed:33059473]
Biofouling. 2020 Sep;36(8):909-921.
This study investigated the effect of the quorum sensing molecules (QSMs) Farnesol, 2-phenylehtanol, tyrosol and tryptophol against planktonic cells, filamentation and biofilms of Sporothrix spp. The antifungal activity of QSMs was evaluated by broth microdilution. QSMs showed MICs in the ranges of 0.01-1 microM (Farnesol), 1-8 mM (2-phenylehtanol and tyrosol), and >16 mM (tryptophol). Filamentous biofilm formation was inhibited by Farnesol and 2-phenylehtanol and stimulated by tyrosol. Yeast biofilm formation was inhibited by 2-phenylehtanol and tyrosol. Tryptophol did not affect Sporothrix biofilm formation. QSMs showed MICs against mature biofilms of 8-32 microM (Farnesol), 8-32 mM (2-phenylehtanol) and 64-128 mM (tyrosol). In conclusion, Farnesol, 2-phenylethanol and tyrosol have antifungal activity against planktonic and sessile cells and modulate filamentation and biofilm formation in Sporothrix spp.
Antifungal activity of farnesol incorporated in liposomes and associated with fluconazole.[Pubmed:33058818]
Chem Phys Lipids. 2020 Nov;233:104987.
Candida infections represent a threat to human health. Candida albicans is the main causative agent of invasive candidiasis, especially in immunosuppressed patients. The emergence of resistant strains has required the development of new therapeutic strategies. In this context, the use of liposomes as drug carrier systems is a promising alternative in drug development. Thus, considering the evidence demonstrating that sesquiterpene Farnesol is a bioactive compound with antifungal properties, this study evaluated the activity Farnesol-containing liposomes against different Candida strains. The IC50 of Farnesol and its liposomal formulation was assessed in vitro using cultures of Candida albicans, Candida tropicalis, and Candida krusei. The Minimum Fungicidal Concentration (MFC) was established by subculture in solid medium. The occurrence of fungal dimorphism was analyzed using optical microscopy. The effects on antifungal resistance to fluconazole were assessed by evaluating the impact of combined therapy on the growth of Candida strains. The characterization of liposomes was carried out considering their vesicular size, polydispersion index, and zeta medium potential, in addition to electron microscopy analysis. Farnesol exerted an antifungal activity that might be associated with the inhibition of fungal dimorphism, especially in Candida albicans. The incorporation of Farnesol into liposomes significantly increased its antifungal activity against C. albicans, C. tropicalis, and C. krusei. In addition, liposomal Farnesol potentiated the action of fluconazole against C. albicans and C. tropicalis. On the other hand, the association of unconjugated Farnesol with fluconazole resulted in antagonistic effects. In conclusion, Farnesol-containing liposomes have the potential to be used in antifungal drug development. However, further research is required to investigate how the antifungal properties of Farnesol are affected by the interaction with liposomes, contributing to the modulation of antifungal resistance to conventional drugs.
Antibiotic potentiation of natural products: A promising target to fight pathogenic bacteria.[Pubmed:32972338]
Curr Drug Targets. 2020 Sep 24. pii: CDT-EPUB-110195.
Pathogenic microorganisms should be considered as human number one foe as witnessed by recent outbreaks of coronavirus disease (COVID-19) and with bacteria no longer sensitive to existing antibiotics. The resistance of pathogenic bacteria and deaths attributable to bacterial infections is increasing exponentially. Bacteria used different mechanisms to counterattack to existing antibiotics namely (i) enzymatic inhibition, (ii) penicillin binding protein modification, (iii) porin mutations, (iv) efflux pumps and (v) molecular modifications of antibiotic targets. Developing new antibiotics would be time consuming to address such situation, thus one of the promising approaches is by potentiating existing antibiotics. Plants used synergism to naturally defend and protect themselves from microbes. Using the same strategy, several studies have shown that the combinations of natural products and antibiotics could effectively prolong the lifespan of existing antibiotics and minimize the impact and emergence of antibiotic resistance. Combining essential oils constituents namely uvaol, ferruginol, Farnesol, carvacrol, with antibiotics have proved to be efficient efflux pump inhibitors. Plant-derived compounds such as gallic acid and tannic acid are effective potentiators of various antibiotics including novobiocin, chlorobiocin, coumermycin, fusidic acid, and rifampicin, resulting in a 4-fold increase in the potencies of these antibiotics. Several lines of research, as discussed in this review, have demonstrated the effectiveness of natural products in potentiating existing antibiotics. For this reason, the search for more efficient combinations should be an ongoing process with the aim to extend the life of the ones that we have and maybe preserve the life for the ones that is yet to come.
Myocardial hypertrophy is prevented by farnesol through oxidative stress and ERK1/2 signaling pathways.[Pubmed:32956645]
Eur J Pharmacol. 2020 Nov 15;887:173583.
Farnesol is a sesquiterpene found in several plants, with multiple pharmacological activities. However, pharmacological actions of Farnesol in the treatment of cardiac hypertrophy are not yet reported. This study aimed to investigate the effect and regulatory mechanisms of Farnesol against isoproterenol-induced pathological cardiac hypertrophy. Male Wistar rats were treated for 8 days with isoproterenol (4.5 mg/kg; i. p.) and with Farnesol (50 muM; i. p.). Hearts were subjected to evaluation of left ventricular developed pressure (LVDP), coronary pressure, electrocardiogram, histopathological analysis, reactive oxygen species (ROS) generation, antioxidant enzyme activity, and pro- and anti-apoptosis protein expression. The results showed that severe impairment of LVDP induced by cardiac hypertrophy was significantly prevented by Farnesol treatment. Moreover, Farnesol attenuated electrocardiographic changes that are characteristic of cardiac hypertrophy, as well as prevented the increase of fibrosis and migration of inflammatory cells in cardiac tissue. Additionally, Farnesol treatment prevented the increase of cardiac ROS generation and restored the activity of endogenous antioxidant enzymes, such as SOD and catalase. It was also evidenced that Farnesol decreased the ERK1/2, Bax and Caspase 3 activation, and an increase of AKT and Bcl-2 protein expression, which can be associated with the pathological cardiac remodeling and also with cardioprotection mediated by Farnesol, respectively. These results suggest that Farnesol is a novel therapeutic agent for amelioration of cardiac hypertrophy in rats.
Dual antibacterial drug-loaded nanoparticles synergistically improve treatment of Streptococcus mutans biofilms.[Pubmed:32853808]
Acta Biomater. 2020 Oct 1;115:418-431.
Dental caries (i.e., tooth decay), which is caused by biofilm formation on tooth surfaces, is the most prevalent oral disease worldwide. Unfortunately, many anti-biofilm drugs lack efficacy within the oral cavity due to poor solubility, retention, and penetration into biofilms. While drug delivery systems (DDS) have been developed to overcome these hurdles and improve traditional antimicrobial treatments, including Farnesol, efficacy is still modest due to myriad resistance mechanisms employed by biofilms, suggesting that synergistic drug treatments may be more efficacious. Streptococcus mutans (S. mutans), a cariogenic pathogen and biofilm forming model organism, has several key virulence factors including acidogenicity and exopolysaccharide (EPS) matrix synthesis. Flavonoids, such as myricetin, can reduce both biofilm acidogenicity and EPS synthesis. Therefore, a nanoparticle carrier (NPC) DDS with flexibility to co-load Farnesol in the hydrophobic core and myricetin within the cationic corona, was tested in vitro using established and developing S. mutans biofilms. Co-loaded NPC treatments effectively disrupted biofilm biomass (i.e., dry weight) and reduced biofilm viability by ~3 log CFU/mL versus single drug-only controls in developing biofilms, suggesting dual-drug delivery exhibits synergistic anti-biofilm effects. Mechanistic studies revealed that co-loaded NPCs synergistically inhibited planktonic bacterial growth compared to controls and reduced S. mutans acidogenicity due to decreased atpD expression, a gene associated with acid tolerance. Moreover, the myricetin-loaded NPC corona enhanced NPC binding to tooth-mimetic surfaces, which can increase drug efficacy through improved retention at the biofilm-apatite interface. Altogether, these findings suggest promise for co-delivery of myricetin and Farnesol DDS as an alternative anti-biofilm treatment to prevent dental caries.
The neonicotinoid thiacloprid causes transcriptional alteration of genes associated with mitochondria at environmental concentrations in honey bees.[Pubmed:32823041]
Environ Pollut. 2020 Nov;266(Pt 1):115297.
Thiacloprid is widely used in agriculture and may affect pollinators. However, its molecular effects are poorly known. Here, we report the global gene expression profile in the brain of honey bee foragers assessed by RNA-sequencing. Bees were exposed for 72 h to nominal concentrations of 25 and 250 ng/bee via sucrose solution. Determined residue concentrations by LC-MS/MS were 0.59 and 5.49 ng/bee, respectively. Thiacloprid exposure led to 5 and 71 differentially expressed genes (DEGs), respectively. Nuclear genes encoding mitochondrial ribosomal proteins and enzymes involved in oxidative phosphorylation, as well as metabolism enzymes and transporters were altered at 5.49 ng/bee. Kyoto Encylopedia of Genes and Genomes (KEGG) analysis revealed that mitochondrial ribosome proteins, mitochondrial oxidative phosphorylation, pyrimidine, nicotinate and nicotinamide metabolism and additional metabolic pathways were altered. Among 21 genes assessed by RT-qPCR, the transcript of Farnesol dehydrogenase involved in juvenile hormone III synthesis was significantly down-regulated. Transcripts of cyp6a14-like and apolipophorin-II like protein, cytochrome oxidase (cox17) and the non-coding RNA (LOC102654625) were significantly up-regulated at 5.49 ng/bee. Our findings indicate that thiacloprid causes transcriptional changes of genes prominently associated with mitochondria, particularly oxidative phosphorylation. This highlight potential effects of this neonicotinoid on energy metabolism, which may compromise bee foraging and thriving populations at environmentally relevant concentrations.
Dissecting Sesquiterpene Profiles of Lemberger Red Wines Using Ex Vivo Tissue Deuterium-Labeling and Comprehensive Two-Dimensional Gas Chromatography-Time-of-Flight-Mass Spectrometry.[Pubmed:32806123]
J Agric Food Chem. 2020 Aug 19;68(33):8936-8941.
By means of ex vivo tissue deuterium-labeling using the stable isotope-labeled precursor [6,6,6-(2)H3]-(+/-)-mevalonolactone and microvinification experiments, we were able to show for the first time that the three sesquiterpene hydrocarbons, guaiazulene, delta-selinene, and selina-3,7(11)-diene, in Lemberger red wines do not originate from acid-catalyzed cyclization of yeast-derived Farnesol and nerolidol. The three aforementioned sesquiterpene hydrocarbons could be unambiguously identified as grape-derived secondary metabolites and can therefore be considered as variety-specific marker compounds. The analysis of sesquiterpene hydrocarbons in red wine samples was performed by solid-phase extraction-headspace solid-phase microextraction-comprehensive two-dimensional gas chromatography-time of flight-mass spectrometry. The developed methodology paves the way for an analytical verification of grape variety labeling in wine authenticity control.


