trans-Beta-Apo-8'-carotenalCAS# 1107-26-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1107-26-2 | SDF | Download SDF |
| PubChem ID | 5478003 | Appearance | Powder |
| Formula | C30H40O | M.Wt | 416.6 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
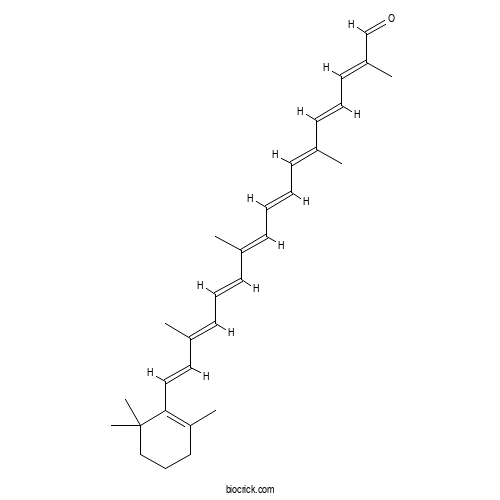
| Chemical Name | (2E,4E,6E,8E,10E,12E,14E,16E)-2,6,11,15-tetramethyl-17-(2,6,6-trimethylcyclohexen-1-yl)heptadeca-2,4,6,8,10,12,14,16-octaenal | ||
| SMILES | CC1=C(C(CCC1)(C)C)C=CC(=CC=CC(=CC=CC=C(C)C=CC=C(C)C=O)C)C | ||
| Standard InChIKey | DFMMVLFMMAQXHZ-DOKBYWHISA-N | ||
| Standard InChI | InChI=1S/C30H40O/c1-24(13-8-9-14-25(2)16-11-18-27(4)23-31)15-10-17-26(3)20-21-29-28(5)19-12-22-30(29,6)7/h8-11,13-18,20-21,23H,12,19,22H2,1-7H3/b9-8+,15-10+,16-11+,21-20+,24-13+,25-14+,26-17+,27-18+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | beta-Apo-8'-carotenal, canthaxanthin and astaxanthin, are inducers of CYP1A1 and 1A2 in the rat. These carotenoids form a new class of inducers of CYP1A, structurally very different from the classical inducers as 3-methylcholanthrene, beta-naphtoflavone or dioxin. | |||||

trans-Beta-Apo-8'-carotenal Dilution Calculator

trans-Beta-Apo-8'-carotenal Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4004 mL | 12.0019 mL | 24.0038 mL | 48.0077 mL | 60.0096 mL |
| 5 mM | 0.4801 mL | 2.4004 mL | 4.8008 mL | 9.6015 mL | 12.0019 mL |
| 10 mM | 0.24 mL | 1.2002 mL | 2.4004 mL | 4.8008 mL | 6.001 mL |
| 50 mM | 0.048 mL | 0.24 mL | 0.4801 mL | 0.9602 mL | 1.2002 mL |
| 100 mM | 0.024 mL | 0.12 mL | 0.24 mL | 0.4801 mL | 0.6001 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (-)-Verbenone
Catalog No.:BCN9980
CAS No.:1196-01-6
- Harmol
Catalog No.:BCN9979
CAS No.:487-03-6
- Henricine
Catalog No.:BCN9978
CAS No.:107783-46-0
- omega-Benzoyl oxyphloracetophenone
Catalog No.:BCN9977
CAS No.:65982-77-6
- AT101
Catalog No.:BCN9976
CAS No.:866541-93-7
- 9-Methyl-9-azabicyclo[3.3.1]nonan-3-one
Catalog No.:BCN9975
CAS No.:552-70-5
- Platycogenin A
Catalog No.:BCN9974
CAS No.:1459719-53-9
- Peltatoside
Catalog No.:BCN9973
CAS No.:23284-18-6
- Terpinyl acetate
Catalog No.:BCN9972
CAS No.:80-26-2
- Vescalagin
Catalog No.:BCN9971
CAS No.:36001-47-5
- Butyric acid
Catalog No.:BCN9970
CAS No.:107-92-6
- Psoromic acid
Catalog No.:BCN9969
CAS No.:7299-11-8
- 4',6,7-Trimethoxyisoflavone
Catalog No.:BCN9982
CAS No.:798-61-8
- (R,S)-Equol
Catalog No.:BCN9983
CAS No.:66036-38-2
- Farnesol
Catalog No.:BCN9984
CAS No.:4602-84-0
- 4'-Methoxyflavanone
Catalog No.:BCN9985
CAS No.:97005-76-0
- 1,3-Diphenyl-2-propen-1-one
Catalog No.:BCN9986
CAS No.:94-41-7
- Quercetin-3'-glucoside
Catalog No.:BCN9987
CAS No.:19254-30-9
- trans-Aconitic acid
Catalog No.:BCN9988
CAS No.:4023-65-8
- 3-Octyl alcohol
Catalog No.:BCN9989
CAS No.:589-98-0
- Phloroglucinol aldehyde triethylether
Catalog No.:BCN9990
CAS No.:59652-88-9
- Daclatasvir
Catalog No.:BCN9991
CAS No.:1009119-64-5
- Geranylacetate
Catalog No.:BCN9992
CAS No.:105-87-3
- Cytochalasin C
Catalog No.:BCN9993
CAS No.:22144-76-9
beta-Carotene and its physiological metabolites: Effects on oxidative status regulation and genotoxicity in in vitro models.[Pubmed:32360219]
Food Chem Toxicol. 2020 Jul;141:111392.
Carotenoids are ubiquitously distributed in nature, beta-carotene being the most frequently found carotenoid in the human diet. In the human body, beta-carotene is absorbed, distributed and metabolized by enzymatic and/or non-enzymatic oxidant cleavage into several metabolites. Despite the broadly accepted biological value of beta-carotene, it has also been considered a double-edged sword, mainly due to its potential antioxidant versus pro-oxidant behaviour. In this sense, the aim of this work was to scrutinize the antioxidant or pro-oxidant potential of beta-carotene and its metabolites, namely trans-beta-apo-8'-carotenal and beta-ionone. Several parameters were evaluated in this study, viz. their effects on reactive species production, both in human whole blood and neutrophils; their effects on lipid peroxidation, in the absence and presence of peroxynitrite anion (ONOO(-)) or hydrogen peroxide (H2O2), using a synaptosomal model; and finally, their putative genotoxic effects in the human hepatic HepG2 cell line. In general, depending on the cellular model and conditions tested, beta-carotene and its metabolites revealed antioxidant effects to varying degrees without significant pro-oxidant or genotoxic effects.
Carotenoid cleavage in chromoplasts of white and yellow-fleshed peach varieties.[Pubmed:30255587]
J Sci Food Agric. 2019 Mar 15;99(4):1795-1803.
BACKGROUND: In peach fruit, carotenoid accumulation in the mesocarp causes the difference between yellow and white genotypes. The latter are generally characterized by a peculiar and more intense aroma, because of higher release of volatiles deriving from dioxygenase-catalysed breakdown of the tetraterpene skeleton. The rate of carotenoid oxidation was investigated in peach (Prunus persica L.) fruits harvested at various stages of development. Two couples of white and yellow-fleshed isogenic varieties and an ancestral white-fleshed genotype were analysed, which had previously shown to differ in Carotenoid Cleavage Dioxygenase 4 allelic composition resulting in various combinations of putatively active/inactive proteins. RESULTS: Carotenoid bleaching activity was localized in the insoluble fraction of fruit flesh chromoplasts. Higher rates of trans-beta-apo-8'-carotenal than beta-carotene bleaching suggest that the first cleavage reaction is the rate-limiting step. Consistently, HPLC analysis did not show the appearance of coloured intermediates in reaction mixtures. High levels of substrate breakdown were found during the initial phases of fruit development in all genotypes examined, whereas significant differences were evident during the second exponential growth phase and ripening onset. Also, the ratio of carotene versus carotenale utilization varied significantly. CONCLUSION: Pattern comparison among activity levels measured in vitro on chromoplast enriched fractions suggests that cleavage enzyme(s) other than Carotenoid Cleavage Dioxygenase 4 play a significant role in carotenoid breakdown during fruit development and ripening. (c) 2018 Society of Chemical Industry.
Single-step preparation of selected biological fluids for the high performance liquid chromatographic analysis of fat-soluble vitamins and antioxidants.[Pubmed:29106966]
J Chromatogr A. 2017 Dec 8;1527:43-52.
Fat-soluble vitamins and antioxidants are of relevance in health and disease. Current methods to extract these compounds from biological fluids mainly need use of multi-steps and multi organic solvents. They are time-consuming and difficult to apply to treat simultaneously large sample number. We here describe a single-step, one solvent extraction of fat-soluble vitamins and antioxidants from biological fluids, and the chromatographic separation of all-trans-retinoic acid, 25-hydroxycholecalciferol, all-trans-retinol, astaxanthin, lutein, zeaxanthin, trans-beta-apo-8'-carotenal, gamma-tocopherol, beta-cryptoxanthin, alpha-tocopherol, phylloquinone, lycopene, alpha-carotene, beta-carotene and coenzyme Q10. Extraction is obtained by adding one volume of biological fluid to two acetonitrile volumes, vortexing for 60s and incubating for 60min at 37 degrees C under agitation. HPLC separation occurs in 30min using Hypersil C18, 100x4.6mm, 5mum particle size column, gradient from 70% methanol+30% H2O to 100% acetonitrile, flow rate of 1.0ml/min and 37 degrees C column temperature. Compounds are revealed using highly sensitive UV-VIS diode array detector. The HPLC method suitability was assessed in terms of sensitivity, reproducibility and recovery. Using the present extraction and chromatographic conditions we obtained values of the fat-soluble vitamins and antioxidants in serum from 50 healthy controls similar to those found in literature. Additionally, the profile of these compounds was also measured in seminal plasma from 20 healthy fertile donors. Results indicate that this simple, rapid and low cost sample processing is suitable to extract fat-soluble vitamins and antioxidants from biological fluids and can be applied in clinical and nutritional studies.
Identification of the Excited-State C horizontal lineC and C horizontal lineO Modes of trans-beta-Apo-8'-carotenal with Transient 2D-IR-EXSY and Femtosecond Stimulated Raman Spectroscopy.[Pubmed:26263319]
J Phys Chem Lett. 2015 May 7;6(9):1592-8.
Assigning the vibrational modes of molecules in the electronic excited state is often a difficult task. Here we show that combining two nonlinear spectroscopic techniques, transient 2D exchange infrared spectroscopy (T2D-IR-EXSY) and femtosecond stimulated Raman spectroscopy (FSRS), the contribution of the C horizontal lineC and C horizontal lineO modes in the excited-state vibrational spectra of trans-beta-apo-8'-carotenal can be unambiguously identified. The experimental results reported in this work confirm a previously proposed assignment based on quantum-chemical calculations and further strengthen the role of an excited state with charge-transfer character in the relaxation pathway of carbonyl carotenoids. On a more general ground, our results highlight the potentiality of nonlinear spectroscopic methods based on the combined use of visible and infrared pulses to correlate structural and electronic changes in photoexcited molecules.
Mechanism of the intramolecular charge transfer state formation in all-trans-beta-apo-8'-carotenal: influence of solvent polarity and polarizability.[Pubmed:25495920]
J Phys Chem B. 2015 Jan 15;119(2):420-32.
In this work we analyzed the infrared and visible transient absorption spectra of all-trans-beta-apo-8'-carotenal in several solvents, differing in both polarity and polarizability at different excitation wavelengths. We correlate the solvent dependence of the kinetics and the band shape changes in the infrared with that of the excited state absorption bands in the visible, and we show that the information obtained in the two spectral regions is complementary. All the collected time-resolved data can be interpreted in the frame of a recently proposed relaxation scheme, according to which the major contributor to the intramolecular charge transfer (ICT) state is the bright 1Bu(+) state, which, in polar solvents, is dynamically stabilized through molecular distortions and solvent relaxation. A careful investigation of the solvent effects on the visible and infrared excited state bands demonstrates that both solvent polarity and polarizability have to be considered in order to rationalize the excited state relaxation of trans-8'-apo-beta-carotenal and clarify the role and the nature of the ICT state in this molecule. The experimental observations reported in this work can be interpreted by considering that at the Franck-Condon geometry the wave functions of the S1 and S2 excited states have a mixed ionic/covalent character. The degree of mixing depends on solvent polarity, but it can be dynamically modified by the effect of polarizability. Finally, the effect of different excitation wavelengths on the kinetics and spectral dynamics can be interpreted in terms of photoselection of a subpopulation of partially distorted molecules.
Dynamics of the time-resolved stimulated Raman scattering spectrum in presence of transient vibronic inversion of population on the example of optically excited trans-beta-apo-8'-carotenal.[Pubmed:24880285]
J Chem Phys. 2014 May 28;140(20):204312.
We have studied the effect of transient vibrational inversion of population in trans-beta-apo-8(')-carotenal on the time-resolved femtosecond stimulated Raman scattering (TR-FSRS) signal. The experimental data are interpreted by applying a quantum mechanical approach, using the formalism of projection operators for constructing the theoretical model of TR-FSRS. Within this theoretical frame we explain the presence of transient Raman losses on the Stokes side of the TR-FSRS spectrum as the effect of vibrational inversion of population. In view of the obtained experimental and theoretical results, we conclude that the excited S2 electronic level of trans-beta-apo-8(')-carotenal relaxes towards the S0 ground state through a set of four vibrational sublevels of S1 state.
Acyclic carotenoid and cyclic apocarotenoid cleavage by an orthologue of lignostilbene-alpha,beta-dioxygenase in Rhodopseudomonas palustris.[Pubmed:23946507]
J Biochem. 2013 Nov;154(5):449-54.
Carotenoid cleavage oxygenases catalyse formation of apocarotenoids and the precursors of phytohormones, abscisic acid and strigolactones through oxidative cleavage at specific double bonds of carotenoids. A gene encoding a presumed bacterial oxygenase homologous to lignostilbene-alpha,beta-dioxygenases has been found in the genome of Rhodopseudomonas palustris. By analysing apocarotenoids in recombinant Escherichia coli strains, it was found that the presumed oxygenase catalyses the 15,15' double bond cleavage of lycopene and neurosporene. Cell lysate containing the recombinant protein cleaved all-trans-beta-apo-8'-carotenal at the 15,15' double bond into retinal and apo-8',15'-apocarotene-dial. These data demonstrate for the first time that the orthologue of lignostilbene-alpha,beta-dioxygenase found in the carotenogenic phototrophic bacterium has the 15,15' double bond cleavage activity towards both the acyclic carotenoids and cyclic apocarotenoid.
Method development and analysis of retail foods and beverages for carotenoid food colouring materials E160a(ii) and E160e.[Pubmed:12627577]
Food Addit Contam. 2003 Feb;20(2):115-26.
An analytical method using high-performance liquid chromatography with photodiode array detection was developed and applied to the determination of the permitted food colour additives beta-carotene(E160a(ii)) and beta-apo-8'-carotenal (E160e) in foods and beverages. The scope of previously reported methods has been broadened to cover a wide range of retail foods and enzymatic hydrolysis has been used in place of saponification for high-fat samples. Quantitative results (greater then 0.1 mg kg(-1)) are given for the major colour principals trans-beta-apo-8'-carotenal and trans-beta-carotene. Semiquantitative results are given for the various cis-isomers of each colorant for which authentic reference standards were not available. The method has been used successfully for the analysis of a wide range of foodstuffs with differing fat content without the need for saponification, except for moderate- to high-fat foodstuffs containing significant levels of emulsifiers, for which it was limited. The results suggest that beta-apo-8'-carotenal (E160e) does not have widespread use in the UK. None of the samples exhibited a total beta-carotene content greater than 20 mg kg(-1) and none of the high-fat samples and only one of the 17 low-fat/beverage samples contained total beta-carotene at levels less than 0.1 mg kg(-1). The total beta-carotene contents of the low-fat/beverage samples ranged from 0.4 +/- 0.03 to 8.4 i 0.71 mg kg(-1), and the total beta-carotene contents of the high-fat samples ranged from 0.1 +/- 0.01 (jelly confectionery) to 18.5 +/- 0.98 mg kg(-1) (processed cheese).


