gamma-DecalactoneCAS# 706-14-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 706-14-9 | SDF | Download SDF |
| PubChem ID | 12813 | Appearance | Oil |
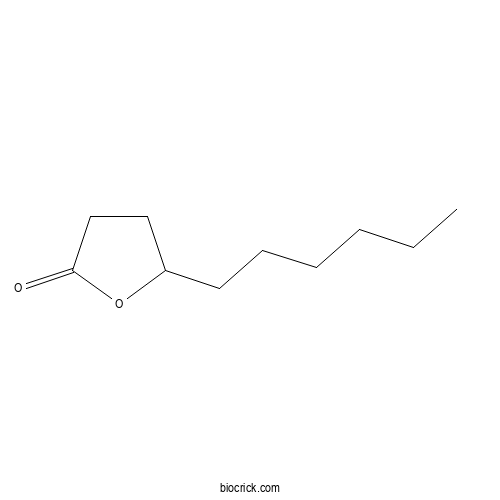
| Formula | C10H18O2 | M.Wt | 170.2 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-hexyloxolan-2-one | ||
| SMILES | CCCCCCC1CCC(=O)O1 | ||
| Standard InChIKey | IFYYFLINQYPWGJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H18O2/c1-2-3-4-5-6-9-7-8-10(11)12-9/h9H,2-8H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | gamma-Decalactone is a valuable aroma compound.could be food industry. | |||||

gamma-Decalactone Dilution Calculator

gamma-Decalactone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.8754 mL | 29.3772 mL | 58.7544 mL | 117.5088 mL | 146.886 mL |
| 5 mM | 1.1751 mL | 5.8754 mL | 11.7509 mL | 23.5018 mL | 29.3772 mL |
| 10 mM | 0.5875 mL | 2.9377 mL | 5.8754 mL | 11.7509 mL | 14.6886 mL |
| 50 mM | 0.1175 mL | 0.5875 mL | 1.1751 mL | 2.3502 mL | 2.9377 mL |
| 100 mM | 0.0588 mL | 0.2938 mL | 0.5875 mL | 1.1751 mL | 1.4689 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,7-Dihydroxy 3,3',4',5',6,8-hexamethoxyflavone
Catalog No.:BCN9881
CAS No.:96887-18-2
- Cymarin
Catalog No.:BCN9880
CAS No.:508-77-0
- Dihydroquinine
Catalog No.:BCN9879
CAS No.:522-66-7
- Tricaprin
Catalog No.:BCN9878
CAS No.:621-71-6
- Lyciumamide B
Catalog No.:BCN9877
CAS No.:1647111-41-8
- 4-Hydroxyisophthalic acid
Catalog No.:BCN9876
CAS No.:636-46-4
- Syringetin
Catalog No.:BCN9875
CAS No.:4423-37-4
- Norharman
Catalog No.:BCN9874
CAS No.:244-63-3
- 2-(4-Hydroxybenzal)acetophenone
Catalog No.:BCN9873
CAS No.:20426-12-4
- Condurango glycoside A
Catalog No.:BCN9872
CAS No.:11051-90-4
- Lappaconine
Catalog No.:BCN9871
CAS No.:23943-93-3
- Cannabiscitrin
Catalog No.:BCN9870
CAS No.:520-14-9
- 2-(2-Hydroxybenzal)acetophenone
Catalog No.:BCN9883
CAS No.:644-78-0
- (1S,2S,5S)-(-)-Myrtanol
Catalog No.:BCN9884
CAS No.:53369-17-8
- 6-Methoxyflavanone
Catalog No.:BCN9885
CAS No.:3034-04-6
- 15-Deoxypulic acid
Catalog No.:BCN9886
CAS No.:95523-05-0
- 4-Oxadocosane-1,2-diol
Catalog No.:BCN9887
CAS No.:544-62-7
- Tribulosin
Catalog No.:BCN9888
CAS No.:79974-46-2
- Cinnamtannin A2
Catalog No.:BCN9889
CAS No.:86631-38-1
- Tryptamine hydrochloride
Catalog No.:BCN9890
CAS No.:343-94-2
- Caffeoyl alcohol
Catalog No.:BCN9891
CAS No.:3598-26-3
- 5-Geranoxy-7-methoxycoumarin
Catalog No.:BCN9892
CAS No.:7380-39-4
- (-)-Linalool
Catalog No.:BCN9893
CAS No.:126-91-0
- Methyl anthranilate
Catalog No.:BCN9894
CAS No.:134-20-3
Comprehensive investigation of lactones and furanones in icewines and dry wines using gas chromatography-triple quadrupole mass spectrometry.[Pubmed:33233229]
Food Res Int. 2020 Nov;137:109650.
A number of lactones and furanones associated with pleasant odorants play a vital role in grape and wine aroma profiles. However, they are usually present at trace levels and are particularly challenging to measure. In this work, an optimized method based on solid-phase extraction (SPE) coupled with gas chromatography-triple quadrupole mass spectrometry (GC-QqQ-MS/MS) was developed for simultaneous determination of 14 lactones and 3 furanones. The validation was carried out using different types of wine as matrices, and satisfactory linearity, sensitivity, trueness and precision were confirmed. Furaneol and sotolon showed significantly lower limits of detection (LODs) in three real wines compared to model wine due to the matrix effect. Furthermore, the method was successfully applied to investigate the concentration range of lactones and furanones in several icewines, dry red and white wines. Icewines contained higher concentrations of most lactones and furanones compared with dry red and white wines. Partial least squares-discriminate analysis (PLS-DA) also indicated that gamma-hexa-, gamma-octa-, gamma-nona-, gamma-deca-, delta-hexa-, and delta-decalactone, as well as 5,6-dihydro-6-pentyl-2H-pyran-2-one (C10 massoia lactone), sotolon and homofuraneol contributed greatly to the discrimination between icewines and dry wines. Moreover, the calculation of odor activity value (OAV) suggested that gamma-octa-, gamma-nona-, and gamma-Decalactone, as well as furaneol and homofuraneol contributed greatest to the aroma of icewines.
Biotransformation of 12-hydroxystearic acid to gamma-decalactone: Comparison of two separation systems.[Pubmed:32890570]
J Microbiol Methods. 2020 Sep 3;178:106041.
Biotransformation of natural products to the natural flavoring, gamma-Decalactone (GDL), has attracted considerable attention. However, improving its yield is challenging due to its high feedback inhibition of yeast cells, which lowers the productivity of the biotransformation process. In this study, we compared two in situ separation processes established by adding either resin (HZ-816) or cyclopentasiloxane (DC345) to a biotransformation medium and investigated their efficiency and effect on yeast metabolism. Compared with a control, yields from the medium with HZ-816 and DC345 increased by 140% and 175%, respectively. However, after 84 h of biotransformation, the protein leakage in the medium with HZ-816 and DC345 was respectively 2.04 times and 1.43 times that of the control. Meanwhile, the mortality of yeast cells was 32.8% and 24.0% in the medium with HZ-816 and DC345, respectively, whereas that in the control was 20.1%. Our findings indicate that a cyclase is involved in the final step of the biotransformation. The activity of the yeast cyclase in the DC345 system was 3.33 times greater than that in the HZ-816 system. The DC345 system was superior to the HZ-816 resin system in this separation process because its yield was 30.8% greater and it had less cellular damage. Thus, we showed that the DC345 system has potential as a new separation system for the production of GDL by biotransformation.
The characteristic smell emitted from two scale insects, Ceroplastes japonicus and Ceroplastes rubens.[Pubmed:32419623]
Biosci Biotechnol Biochem. 2020 Aug;84(8):1541-1545.
The volatile components emitted from two scale insects, Ceroplastes japonicus and Ceroplastes rubens, were identified using GC-MS analysis. The major volatile components of the solvent extract from C. japonicus were alpha-humulene (35.8%) and delta-cadinene (17.0%), while those of C. rubens were beta-selinene (10.3%) and beta-elemene (5.1%). In GC/olfactometry, linalool, butyric acid, 3-methylbutyric acid, 2-methylbutyric acid, and vanillin were identified as the odor-active components of the extract from C. japonicus, in addition to trace amounts of trans-4,5-epoxy-(2E)-decenal, 4-methyl-(3E)-hexenoic acid, and phenylacetic acid. With regard to C. rubens, trans-4,5-epoxy-(2E)-decenal, 3-methylbutyric acid, and phenylacetic acid were identified as the odor-active components. Besides, decan-1,4-olide (gamma-Decalactone) with milky cherry-like note and 3-hydroxy-4,5-dimethylfuran-2(5H)-one (sotolone) with brown sugar-like note were also detected as the characteristic cherry-like sweet-and-sour note of these two scale insects. ABBREVIATIONS: GC: Gas chromatography; GC/O: gas chromatography/olfactometry.
Differences in PpAAT1 Activity in High- and Low-Aroma Peach Varieties Affect gamma-Decalactone Production.[Pubmed:32001520]
Plant Physiol. 2020 Apr;182(4):2065-2080.
Aroma contributes to the unique flavors of fruits and is important for fruit quality evaluation. Among the many volatiles in peach (Prunus persica) fruits, gamma-Decalactone has the greatest contribution to the characteristic peach aroma. Some peach cultivars have gamma-Decalactone contents that are too low to detect. Comparison of the transcriptomes and metabolomes of a high-aroma cultivar, Fenghuayulu, and a low-aroma cultivar, Achutao, suggested that amino acid substitutions in ALCOHOL ACYLTRANSFERASE (PpAAT1) are responsible for the undetectable levels of gamma-Decalactone in cv Achutao fruit. Modeling and molecular docking analysis of PpAAT1 indicated that the substituted residues might determine substrate recognition or act as control channels to the active site. In vitro enzyme assays on PpAAT1 heterologously expressed and purified from Escherichia coli and in vivo assays using transient PpAAT1 expression in Nicotiana benthamiana or the oleaginous yeast Yarrowia lipolytica indicated that PpAAT1 from high-aroma cultivars was more efficient than PpAAT1 from low-aroma cultivars in catalyzing the conversion of 4-hydroxydecanoyl-coenzyme A into gamma-Decalactone. Examination of loss-of-function mutations of PpAAT1 generated by CRISPR/Cas9 in cv Fenghuayulu showed that fruits with PpAAT1 mutations had significantly lower gamma-Decalactone contents. Expression of the version of PpAAT1 from cv Fenghuayulu in cv Achutao restored gamma-Decalactone levels to those measured in 'Fenghuayulu', confirming the specific contribution of PpAAT1 to the formation of this key aroma compound. These results show how the biosynthesis of the peach aroma compound gamma-Decalactone is compromised in some low-aroma cultivars and illustrate the physiological role of PpAAT1 in plant lactone biosynthesis.
Characterization of Aroma-Active Compounds in Four Yeast Extracts Using Instrumental and Sensory Techniques.[Pubmed:31833769]
J Agric Food Chem. 2020 Jan 8;68(1):267-278.
Gas chromatography-olfactometry coupled with sensory analysis and partial least-squares regression (PLSR) analysis led to the identification of the odorants responsible for the different flavors of four yeast extracts. Sensory analysis showed that LA00L had an intense sulfurous attribute, and LA00 was characterized by fatty and green notes, FA31 exhibited the floral odor, while KA02 had strong phenolic, animal, fermented, roasted, and caramellic notes. A total of 37 key aroma compounds with odor activity values greater than 1 were determined. 2,4-Di-tert-butylphenol and methional were the most potent aroma compounds. In addition, the key aroma compounds in LA00L were nonanal, dimethyl disulfide, and gamma-Decalactone. Octanal, dimethyl disulfide, and benzeneacetaldehyde were the key aroma compounds in LA00. In FA31, styrene, benzeneacetaldehyde, and acetophenone were the key aroma compounds, while indole, 2-methoxyphenol, benzeneacetaldehyde, and p-cresol contributed significantly to the aroma of KA02. PLSR showed that p-cresol and indole were significantly responsible for the phenolic and animal notes inducing the off-flavor (yeasty odor) of yeasty extracts. More significantly, indole was first reported to have an important effect on yeasty odor.
Citric acid treatment reduces decay and maintains the postharvest quality of peach (Prunus persica L.) fruit.[Pubmed:31763012]
Food Sci Nutr. 2019 Sep 27;7(11):3635-3643.
Peaches are easily perishable fruit, and their quality is quickly lost after harvest. In this study, "Hujingmilu" peach (Prunus persica L.) fruit was treated with citric acid (CA) and stored at 20 degrees C for 15 days. Fruit decay and quality were evaluated during the storage period. Compared with the control, CA treatment did not inhibit climacteric ethylene release, but CA was significantly effective at maintaining firmness, inhibiting decay, and preventing a decrease in titration acid (TA). CA treatment inhibited the increase in total soluble solids (TSS), sucrose, and fructose in the first week after fruit harvest, but then their content was significantly higher in CA-treated fruits than that in the control group. The decrease in malic acid and citric acid was significantly prevented by CA treatment. During storage, the concentrations of C6 volatile compounds decreased rapidly whereas lactones sharply increased, and the concentrations of delta-decalactone, gamma-Decalactone, and gamma-dodecalactone were found to be significantly high in CA fruits compared with the control after the eighth day of storage (p < .05). Similarly, higher contents of chlorogenic acid, neochlorogenic acid, catechin, and L-epicatechin were maintained in fruits treated with CA during the same storage period (p < .05). Our findings suggest that treatment with 10 g/L citric acid can reduce postharvest decay and effectively maintain the texture, flavor, and nutrition quality of peach fruit.
Predominant accumulation of a 3-hydroxy-gamma-decalactone in the male rectal gland complex of the Japanese orange fly, Bactrocera tsuneonis.[Pubmed:31516064]
Biosci Biotechnol Biochem. 2020 Jan;84(1):25-30.
The Japanese orange fly, Bactrocera tsuneonis, infests various citrus crops. While male pheromone components accumulated in the rectal glands are well characterized for Bactrocera, but information regarding the chemical factors involved in the life cycles of B. tsuneonis remains scarce. Herein, several volatile chemicals including a gamma-Decalactone, (3R,4R)-3-hydroxy-4-decanolide [(3R,4R)-HD], were identified as major components, along with acetamide and spiroketals as minor components in the rectal gland complexes of male B. tsuneonis flies. The lactone (3R,4R)-HD was also identified in female rectal gland complexes. The amount of this compound in mature males was significantly higher than those observed in females and immature males. The lactone (3R,4R)-HD was detected in flies fed with sucrose only, indicating that this lactone is not derived from dietary sources during adulthood, but biosynthesized in vivo. The predominant accumulation of (3R,4R)-HD in mature males also suggests a possible role in reproductive behavior.
Enhanced Biotransformation Productivity of Gamma-Decalactone from Ricinoleic Acid Based on the Expanded Vermiculite Delivery System.[Pubmed:31337188]
J Microbiol Biotechnol. 2019 Jul 28;29(7):1071-1077.
Natural gamma-Decalactone (GDL) produced by biotransformation is an essential food additive with a peach-like aroma. However, the difficulty of effectively controlling the concentration of the substrate ricinoleic acid (RA) in water limits the biotransformation productivity, which is a bottleneck for industrialization. In this study, expanded vermiculite (E-V) was utilized as a carrier of RA to increase its distribution in the medium. E-V and three commonly used organic compounds were compared with respect to their effects on the biotransformation process, and the mechanism was revealed. Scanning electron microscopy, Fourier transform infrared spectroscopy and thermogravimetric analysis indicated that RA was physically adsorbed onto the surface of and inside E-V instead of undergoing a chemical reaction, which increased the opportunity for interactions between microorganisms and the substrate. The highest concentration of GDL obtained in the medium with E-V was 6.2 g/l, which was 50% higher than that in the reference sample. In addition, the presence of E-V had no negative effect on the viability of the microorganisms. This study provides a new method for producing natural GDL through biotransformation on an industrial scale.
PON1 increases cellular DNA damage by lactone substrates.[Pubmed:31209508]
Arch Toxicol. 2019 Jul;93(7):2035-2043.
Paraoxonase 1 (PON1) is a high-density lipoprotein (HDL)-associated enzyme that by hydrolysing exogenous and endogenous substrates can provide protection against substrate induced toxicity. To investigate the extent to which PON1 provides protection against lactone induced DNA damage, DNA damage was measured in HepG2 cells using the neutral Comet assay following lactone treatment in the presence and absence of exogenous recombinant PON1 (rPON1). Low dose lactones (10 mM) caused little or no damage while high doses (100 mM) induced DNA damage in the following order of potency: alpha-angelica lactone > gamma-butyrolactone ~ gamma-hexalactone > gamma-heptalactone ~ gamma-octaclactone ~ gamma-furanone ~ gamma-valerolactone > gamma-Decalactone. Co-incubation of 100 mM lactone with rPON1, resulted in almost all cells showing extensive DNA damage, particularly with those lactones that decreased rPON1 activity by > 25%. In contrast, with the lactones that are poor rPON1 subtrates (gamma-Decalactone and gamma-furanone), rPON1 did not increase DNA damage. DNA damage induced by a 1 h co-treatment with 10 mM alpha-angelica lactone and rPON1 was reduced when cells when incubated for a further 4 h in fresh medium suggesting break formation was due to induced DNA damage rather than apoptosis. Preincubation (1-6 h) of alpha-angelica lactone with rPON1 in the absence of cells, decreased cellular DNA damage by around 40% in comparison to cells treated without preincubation. These results suggest that in addition to its well-recognised detoxification effects, PON1 can increase genotoxicity potentially by hydrolysing certain lactones to reactive intermediates that increase DNA damage via the formation of DNA adducts.
Effects of cooling rate on retrograded nucleation of different rice starch-aromatic molecule complexes.[Pubmed:31126450]
Food Chem. 2019 Oct 1;294:179-186.
The effects of cooling rate (CR) on retrograded nucleation of rice starch-aromatic molecule complexes were evaluated. Six aromatic molecules (hexanal, 1-octen-3-ol, gamma-Decalactone, guaiacol, 2, 3-butanedione, and 2-acetyl-1-pyrroline) were chosen to represent the typical aromas in cooked rice. Differential scanning calorimetry results showed that increased CR from 0.34 to 3.04 degrees C/min led to reduced enthalpy change (DeltaH) (in 2-acetyl-1-pyrroline from 2.08 to 1.40J/g), reduced Tp (in 2-acetyl-1-pyrroline 100.91 to 98.29 degrees C whereas in 2,3-butanedione it remained almost constant) and increased Tc-To (but fluctuating in gamma-Decalactone) (p<0.05). These results indicate that the nucleation forming at higher CR were more thermally unstable, less perfect, and more heterogeneous. X-ray diffraction analysis further indicated that nucleation forming at high CR was looser and with lower relative crystallinity (in 2-acetyl-1-pyrroline decreased from 2.18% to 1.00%) (p<0.05). These results may aid the development of procedures for more effective preservation of aromatic molecules in cooked rice.


