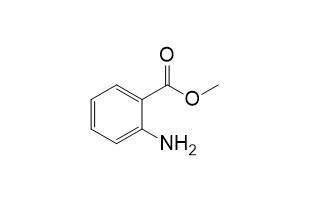
Methyl anthranilateCAS# 134-20-3 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 134-20-3 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C8H9NO2 | M.Wt | 151.1 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Methyl anthranilate is a potent attractant for four species of flower thrips, Thrips hawaiiensis, T. coloratus, T. flavus, and Megalurothrips distalis, irrespective of sex. It also could be used as an additional marker in the determination of citrus honey origin. | |||||

Methyl anthranilate Dilution Calculator

Methyl anthranilate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.6181 mL | 33.0907 mL | 66.1813 mL | 132.3627 mL | 165.4533 mL |
| 5 mM | 1.3236 mL | 6.6181 mL | 13.2363 mL | 26.4725 mL | 33.0907 mL |
| 10 mM | 0.6618 mL | 3.3091 mL | 6.6181 mL | 13.2363 mL | 16.5453 mL |
| 50 mM | 0.1324 mL | 0.6618 mL | 1.3236 mL | 2.6473 mL | 3.3091 mL |
| 100 mM | 0.0662 mL | 0.3309 mL | 0.6618 mL | 1.3236 mL | 1.6545 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (-)-Linalool
Catalog No.:BCN9893
CAS No.:126-91-0
- 5-Geranoxy-7-methoxycoumarin
Catalog No.:BCN9892
CAS No.:7380-39-4
- Caffeoyl alcohol
Catalog No.:BCN9891
CAS No.:3598-26-3
- Tryptamine hydrochloride
Catalog No.:BCN9890
CAS No.:343-94-2
- Cinnamtannin A2
Catalog No.:BCN9889
CAS No.:86631-38-1
- Tribulosin
Catalog No.:BCN9888
CAS No.:79974-46-2
- 4-Oxadocosane-1,2-diol
Catalog No.:BCN9887
CAS No.:544-62-7
- 15-Deoxypulic acid
Catalog No.:BCN9886
CAS No.:95523-05-0
- 6-Methoxyflavanone
Catalog No.:BCN9885
CAS No.:3034-04-6
- (1S,2S,5S)-(-)-Myrtanol
Catalog No.:BCN9884
CAS No.:53369-17-8
- 2-(2-Hydroxybenzal)acetophenone
Catalog No.:BCN9883
CAS No.:644-78-0
- gamma-Decalactone
Catalog No.:BCN9882
CAS No.:706-14-9
- 3-Octanone
Catalog No.:BCN9895
CAS No.:106-68-3
- Decanoic acid
Catalog No.:BCN9896
CAS No.:334-48-5
- 3',4'-Dimethoxyflavone
Catalog No.:BCN9897
CAS No.:4143-62-8
- Dihydroisoferulic acid
Catalog No.:BCN9898
CAS No.:1135-15-5
- cis-Jasmone
Catalog No.:BCN9899
CAS No.:488-10-8
- Methyl benzoate
Catalog No.:BCN9900
CAS No.:93-58-3
- Bufotenine
Catalog No.:BCN9901
CAS No.:487-93-4
- 3-Methyl ellagic acid
Catalog No.:BCN9902
CAS No.:51768-38-8
- 5-Methyl-3-heptanone
Catalog No.:BCN9903
CAS No.:541-85-5
- 3-O-Acetyl 9,11-dehydro beta-boswellic acid
Catalog No.:BCN9904
CAS No.:122651-20-1
- Eupalitin
Catalog No.:BCN9905
CAS No.:29536-41-2
- Eupalitin 3-galactoside
Catalog No.:BCN9906
CAS No.:35399-32-7
Combined effects of Pseudomonas quinolone signal-based quorum quenching and graphene oxide on the mitigation of biofouling and improvement of the application potential for the thin-film composite membrane.[Pubmed:33162137]
Sci Total Environ. 2020 Oct 29:143348.
Biofouling caused by the growth of the biofilm is the main bottleneck that limits the effective operation of thin-film composite (TFC) membrane in the forward osmosis (FO) process. This study investigated the combined effects of graphene oxide (GO) immobilized thin-film nanocomposite (TFN-S) membrane and Pseudomonas quinolone signal (PQS)-based quorum quenching on biofouling mitigation, especially under the operation of pressure-retarded osmosis (PRO) mode, and the influence of Methyl anthranilate (MA) inhibitor on the composition and structure of biofilm was also evaluated. Synthetic wastewater was used as the feed solution, in which the model strain Pseudomonas aeruginosa was added to simulate biofouling. The results showed that GO modification and MA addition both efficiently mitigated flux decline and EPS secretion, but the interference of PQS pathway on biofouling control was better than GO embedding. TFN-S membrane with MA addition exhibited superior anti-biofouling performance based on the combined effects of GO and MA. The alleviated concentration polarization and enhanced hydrophilicity of the TFN-S membrane reduced the flux decline in the early stage. Additionally, the antibacterial property of GO inhibited the viability of the attached bacteria (under PRO mode) and MA further mitigated the EPS secretion and biofilm development in the later stage. In the presence of PQS inhibitor MA, live/total cells ratio was 15% and 13% higher than that of TFC membrane in FO and PRO modes, respectively. Furthermore, exogenous addition of MA led to a relatively loose biofilm structure, resulting in high membrane permeability in the biofouling formation process.
Development of an analytical methodology based on fabric phase sorptive extraction followed by gas chromatography-tandem mass spectrometry to determine UV filters in environmental and recreational waters.[Pubmed:33117984]
Anal Chim Acta X. 2019 Dec 17;4:100038.
A novel method based on fabric phase sorptive extraction (FPSE) followed by gas chromatography-tandem mass spectrometry (GC-MS/MS) has been validated for the simultaneous determination of 11 UV filters (ethylhexyl salicylate, benzyl salicylate, homosalate, benzophenone-3, isoamylmethoxycinnamate, 4-methylbenzylidenecamphor, Methyl anthranilate, etocrylene, 2-ethylhexylmethoxycinnamate, 2-ethylhexyl p-dimethylaminobenzoate, and octocrylene), in natural and recreational waters. Major experimental parameters affecting FPSE procedure have been optimized to obtain the highest extraction efficiency. Different types and sizes of sol-gel coated FPSE media, sample volume, extraction time, and type and volume of desorption solvent were evaluated. The optimal conditions involved the use of a (2.0 x 2.5) cm(2) FPSE device with PDMS based coating for the extraction of 20 mL of water for 20 min. The quantitative desorption of the target compounds was performed with 0.5-1 mL of ethyl acetate. The method was satisfactorily validated in terms of linearity, precision, repeatability and reproducibility. Recovery studies were performed at different concentration levels in real water matrices to show its suitability, obtaining mean values about 90% and satisfactory precision. LODs were at the low ng L(-1) in all cases. Finally, the validated FPSE-GC-MS/MS method was applied to different real samples, including environmental water (lake, river, seawater) and recreational water (swimming-pool), where 8 out of the 11 studied compounds were detected at concentrations between 0.12-123 mug L(-1). FPSE is proposed as an efficient and simple alternative to other extraction and microextraction techniques for the analysis of UV filters in waters. Since no matrix effects were observed, quantification could be carried out by conventional calibration with standard solutions, without the need to perform the complete FPSE procedure, thus allowing a higher throughput in comparison with other microextraction techniques.
Characterization of Volatile Organic Compounds Emitted from Endophytic Burkholderia cenocepacia ETR-B22 by SPME-GC-MS and Their Inhibitory Activity against Various Plant Fungal Pathogens.[Pubmed:32824884]
Molecules. 2020 Aug 19;25(17). pii: molecules25173765.
The use of antagonistic microorganisms and their volatile organic compounds (VOCs) to control plant fungal pathogens is an eco-friendly and promising substitute for chemical fungicides. In this work, endophytic bacterium ETR-B22, isolated from the root of Sophora tonkinensis Gagnep., was found to exhibit strong antagonistic activity against 12 fungal pathogens found in agriculture. Strain ETR-B22 was identified as Burkholderia cenocepacia based on 16S rRNA and recA sequences. We evaluated the antifungal activity of VOCs emitted by ETR-B22. The VOCs from strain ETR-B22 also showed broad-spectrum antifungal activity against 12 fungal pathogens. The composition of the volatile profiles was analyzed based on headspace solid phase microextraction (HS-SPME) gas chromatography coupled to mass spectrometry (GC-MS). Different extraction strategies for the SPME process significantly affected the extraction efficiency of the VOCs. Thirty-two different VOCs were identified. Among the VOC of ETR-B22, dimethyl trisulfide, indole, Methyl anthranilate, methyl salicylate, methyl benzoate, benzyl propionate, benzyl acetate, 3,5-di-tert-butylphenol, allyl benzyl ether and nonanoic acid showed broad-spectrum antifungal activity, and are key inhibitory compounds produced by strain ETR-B22 against various fungal pathogens. Our results suggest that the endophytic strain ETR-B22 and its VOCs have high potential for use as biological controls of plant fungal pathogens.
The missing NH stretch fundamental in S1 methyl anthranilate: IR-UV double resonance experiments and local mode theory.[Pubmed:32568351]
Phys Chem Chem Phys. 2020 Jul 7;22(25):14077-14087.
The infrared spectra of jet-cooled Methyl anthranilate (MA) and the MA-H2O complex are reported in both S0 and S1 states, recorded using fluorescence-dip infrared (FDIR) spectroscopy under jet-cooled conditions. Using a combination of local mode CH stretch modeling and scaled harmonic vibrational character, a near-complete assignment of the infrared spectra is possible over the 1400-3700 cm(-1) region. While the NH stretch fundamentals are easily observed in the S0 spectrum, in the S1 state, the hydrogen bonded NH stretch shift is not readily apparent. Scaled harmonic calculations predict this fundamental at just below 2900 cm(-1) with an intensity around 400 km mol(-1). However, the experimental spectrum shows no evidence of this transition. A local mode theory is developed in which the NH stretch vibration is treated adiabatically. Minimizing the energy of the corresponding stretch state with one quantum of excitation leads to a dislocation of the H atom where there is equal sharing between N and O atoms. The sharing occurs as a result of significant molecular arrangement due to strong coupling of this NH stretch to other internal degrees of freedom and in particular to the contiguous HNC bend. A two-dimensional model of the coupling between the NH stretch and this bend highlights important nonlinear effects that are not captured by low order vibrational perturbation theory. In particular, the model predicts a dramatic dilution of the NH stretch oscillator strength over many transitions spread over more than 1000 cm(-1), making it difficult to observe experimentally.
A strawberry accession with elevated methyl anthranilate fruit concentration is naturally resistant to the pest fly Drosophila suzukii.[Pubmed:32484826]
PLoS One. 2020 Jun 2;15(6):e0234040.
During the past decade, Drosophila suzukii has established itself as a global invasive fruit pest, enabled by its ability to lay eggs into fresh, ripening fruit. In a previous study, we investigated the impact of different strawberry accessions on the development of D. suzukii eggs, in the search of natural resistance. We identified several accessions that significantly reduced adult fly emergence from infested fruit. In the present study, we aimed at understanding the chemical basis of this effect. We first noted that one of the more resistant accessions showed an unusual enrichment of Methyl anthranilate within its fruit, prompting us to investigate this fruit compound as a possible cause limiting fly development. We found that Methyl anthranilate alone triggers embryo lethality in a concentration-dependent manner, unlike another comparable organic fruit compound. We also showed that a chemical fraction of the resistant strawberry accession that contains Methyl anthranilate carries some activity toward the egg hatching rate. Surprisingly, in spite of the lethal effect of this compound to their eggs, adult females are not only attracted to Methyl anthranilate at certain concentrations, but they also display a concentration-dependent preference to lay on substrates enriched in Methyl anthranilate. This study demonstrates that Methyl anthranilate is a potent agonist molecule against D. suzukii egg development. Its elevated concentration in a specific strawberry accession proven to reduce the fly development may explain, at least in part the fruit resistance. It further illustrates how a single, natural compound, non-toxic to humans could be exploited for biological control of a pest species.
A key 'foxy' aroma gene is regulated by homology-induced promoter indels in the iconic juice grape 'Concord'.[Pubmed:32337050]
Hortic Res. 2020 Apr 18;7:67.
'Concord', the most well-known juice grape with a parentage of the North American grape species Vitis labrusca L., possesses a special 'foxy' aroma predominantly resulted from the accumulation of Methyl anthranilate (MA) in berries. This aroma, however, is often perceived as an undesirable attribute by wine consumers and rarely noticeable in the common table and wine grape species V. vinifera. Here we discovered homology-induced promoter indels as a major genetic mechanism for species-specific regulation of a key 'foxy' aroma gene, anthraniloyl-CoA:methanol acyltransferase (AMAT), that is responsible for MA biosynthesis. We found the absence of a 426-bp and/or a 42-bp sequence in AMAT promoters highly associated with high levels of AMAT expression and MA accumulation in 'Concord' and other V. labrusca-derived grapes. These promoter variants, all with direct and inverted repeats, were further confirmed in more than 1,300 Vitis germplasm. Moreover, functional impact of these indels was validated in transgenic Arabidopsis. Superimposed on the promoter regulation, large structural changes including exonic insertion of a retrotransposon were present at the AMAT locus in some V. vinifera grapes. Elucidation of the AMAT genetic regulation advances our understanding of the 'foxy' aroma trait and makes it genetically trackable and amenable in grapevine breeding.
Synthesis and Biological Activities of Some New Benzotriazinone Derivatives Based on Molecular Docking; Promising HepG2 Liver Carcinoma Inhibitors.[Pubmed:32258913]
ACS Omega. 2020 Mar 19;5(12):6781-6791.
In one-pot strategy, diazotization of Methyl anthranilate 5 followed by addition of amino acid ester hydrochloride, we have prepared methyl-2-(4-oxobenzotriazin-3(4H)-yl)alkanoates 6a-c. Starting with hydrazides 7a,b, N-alkyl-2-(4-oxobenzotriazin-3(4H)-yl)alkanamides 9-10(a-h) and methyl-2-(2-(4-oxobenzotriazin-3(4H)-yl)alkanamido)alkanoates 11-12(a-e) were prepared via azide coupling. Hydrazones 13-15 were prepared via condensation of hydrazides 7a,b with 4-methoxybenzaldehyde, 4-dimethylaminobenzaldehyde, and/or arabinose. Molecular docking was done for synthesized compounds using MOE 2008-10 software. The compounds 9a, 12a, 12c, 13a, 13b, and 14b have the most pronounced strong binding affinities toward the target E. coli Fab-H receptor, whereas compounds 3, 11e, 12e, and 13a have the most pronounced strong binding affinities toward the target vitamin D receptor. The in vitro antibacterial activities of the highest binding affinity docked compounds were tested against E. coli, Staphylococcus aureus, and Salmonella spp. Majority of the tested compounds showed effective positive results against E. coli, while they were almost inactive against Staphylococcus aureus and Salmonella spp . The in vitro cytotoxic activities of the highest binding affinity-docked compounds were tested against the human liver carcinoma cell line (HepG2). Some compounds showed potent cytotoxic activity with low IC50 values, especially for 3 (6.525 muM) and 13a (10.97 muM) than that for standard drug doxorubicin (2.06 muM).
Oleanane Triterpenoids from the Leaves of Gymnema inodorum and Their Insulin Mimetic Activities.[Pubmed:32237726]
J Nat Prod. 2020 Apr 24;83(4):1265-1274.
During an effort to find insulin mimetic compounds, the leaves of Gymnema inodorum were shown to have a stimulatory effect on glucose uptake in 3T3-L1 adipocyte cells. Bioassay-guided fractionation on a 70% ethanol extract of G. inodorum was applied to yield two new (1 and 2) and two known (8 and 9) oleanane triterpenoids with a Methyl anthranilate moiety together with five further new oleanane triterpenoids (3-7). The chemical structures of all isolates were determined based on their spectroscopic data, including IR, UV, NMR, and mass spectrometric analysis. The isolated compounds (1-9) were determined for their stimulatory activities on glucose uptake in differentiated 3T3-L1 adipocyte cells using 2-deoxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]-d-glucose (2-NBDG) as a fluorescent-tagged glucose probe. Three compounds (3, 5, and 9) showed stimulatory effects on the uptake of 2-NBDG in 3T3-L1 adipocyte cells. Chemicals with a Methyl anthranilate moiety have been considered as crucial contributors of flavor odor in foods, and quantitative analysis showed the content of compound 8 to be 0.90 +/- 0.01 mg/g of the total extract. These results suggest that the leaves of G. inodorum have the potential to be used as an antidiabetic functional food or tea.
Microwave Assisted Synthesis And Molecular Docking Studies Of Some 4 - (3h)-Quinazolinone Derivatives As Inhibitors Of Human Gamma-Aminobutyric Acid Receptor, The Gaba (A)R-Beta3 Homopentamer.[Pubmed:31840612]
Med Chem. 2019 Dec 16. pii: MC-EPUB-102995.
BACKGROUND: Quinazolines and quinazolinones constitute a major class of biologically active molecules both from natural and synthetic sources. The quinazolinone moiety is an important pharmacophore showing many types of pharmacological activities as shown in recent exhaustive review on the chemistry of 2-heteroaryl & heteroalkyl-4-quinazolinones4-quinazolinones are the formal condensation products of anthranilic acid and amides, and they can also be prepared in this fashion through the Niementowski quinazolinone synthesis, named after it's discoverer Stefan Niementowski. Quinazoline and condensed Quinazoline exhibit potent central nervous system (CNS) activities like anti-anxiety, analgesic, anti-inflammatory [10] and anticonvulsant [11]. Quinazolin-4-ones with 2, 3-disubstitution is reported to possess significant analgesic, anti-inflammatory and anticonvulsant activities Methods: To expand these views and application profiles, efforts have been developed for the synthesis of a new class of quinazolinone by incorporating different amines into synthesized benzoxazinone ring by replacing O atom in the ring. Up to now, a great number of various procedures have been proposed for the synthesis of quinazolin-4-ones in the past few years [16]. Using microwave radiation, this reaction could be easily and rapidly performed in very good yields, providing a large quantity of various 3-substituted-2- propyl-quinazolin-4-one derivatives which can be employed as useful bioactive compounds. We report a facile and efficient method for the synthesis of 3-substituted-2-propyl-quinazolin-4-one by the condensation reaction of Anthranilic acid or Halogen substituted anthranilic acid or Methyl anthranilate, butanoic anhydride with various amines. we also reports a drug/ligand or receptor/protein interactions by identifying the suitable active sites in human gamma-aminobutyric acid receptor, the gaba (a)r-beta3 homopentamer human gamma-aminobutyric acid receptor, the gaba (a)r-beta3 homopentamer protein. RESULTS: We are pleased to find that the reaction provided of 3-alkyl/aryl-2-alkyl-quinazolin-4-one gives good yield as well as good quality of product by using MW. All the synthesized compounds were subjected to grid-based molecular docking studies. The results shows that compound 4t have good affinity to the active site residue of human gamma-aminobutyric acid receptor, the gaba (a)r-beta3 homopentamer. CONCLUSION: The Microwave irradiation for synthesis of the title compounds offers reduction in reaction time, operation simplicity, cleaner reaction, easy work up and improved yields. The procedure clearly highlights the advantages of Green Chemistry. The data reported in this article may be a helpful guide for the medicinal chemists who are working in this area. The Protein-Ligand interaction plays a significant role in structural based drug designing. In the Present work we have docked the ligand, 2, 3-disubstituted quinazolinone with the proteins that are used as the target for GABA-A receptor.
Methyl anthranilate: A novel quorum sensing inhibitor and anti-biofilm agent against Aeromonas sobria.[Pubmed:31703863]
Food Microbiol. 2020 Apr;86:103356.
Quorum sensing (QS), bacterial cell-to-cell communication, is a gene regulatory mechanism that regulates virulence potential and biofilm formation in many pathogens. Aeromonas sobria, a common aquaculture pathogen, was isolated and identified by our laboratory from the deteriorated turbot, and its potential for virulence factors and biofilm production was regulated by QS system. In view of the interference with QS system, this study was aimed to investigate the effect of Methyl anthranilate at sub-Minimum Inhibitory Concentrations (sub-MICs) on QS-regulated phenotypes in A. sobria. The results suggested that 0.5muL/mL of Methyl anthranilate evidently reduced biofilm formation (51.44%), swinging motility (74.86%), swarming motility (71.63%), protease activity (43.08%), and acyl-homoserine lactone (AHL) production. Furthermore, the real-time quantitative PCR (RT-qPCR) and in silico analysis showed that Methyl anthranilate might inhibit QS system in A. sobria by interfering with the biosynthesis of AHL, as well as competitively binding with receptor protein. Therefore, our data indicated the feasibility of Methyl anthranilate as a promising QS inhibitor and anti-biofilm agent for improving food safety.
Vibronic spectroscopy of methyl anthranilate and its water complex: hydrogen atom dislocation in the excited state.[Pubmed:31531502]
Phys Chem Chem Phys. 2019 Oct 14;21(38):21355-21369.
Laser-induced fluorescence (LIF) excitation, dispersed fluorescence (DFL), UV-UV-hole burning, and UV-depletion spectra have been collected on Methyl anthranilate (MA, methyl 2-aminobenzoate) and its water-containing complex (MA-H2O), under jet-cooled conditions in the gas phase. As a close structural analog of a sunscreen agent, MA has a strong absorption due to the S0-S1 transition that begins in the UV-A region, with the electronic origin at 28 852 cm(-1) (346.6 nm). Unlike most sunscreens that have fast non-radiative pathways back to the ground state, MA fluoresces efficiently, with an excited state lifetime of 27 ns. Relative to methyl benzoate, inter-system crossing to the triplet manifold is shut off in MA by the strong intramolecular NHO[double bond, length as m-dash]C H-bond, which shifts the (3)npi* state well above the (1)pipi* S1 state. Single vibronic level DFL spectra are used to obtain a near-complete assignment of the vibronic structure in the excited state. Much of the vibrational structure in the excitation spectrum is Franck-Condon activity due to three in-plane vibrations that modulate the distance between the NH2 and CO2Me groups, nu33 (421 cm(-1)), nu34 (366 cm(-1)), and nu36 (179 cm(-1)). Based on the close correspondence between experiment and theory at the TD-DFT B3LYP-D3BJ/def2TZVP level of theory, the major structural changes associated with electronic excitation are evaluated, leading to the conclusion that the major motion is a reorientation and constriction of the 6-membered H-bonded ring closed by the intramolecular NHO[double bond, length as m-dash]C H-bond. This leads to a shortening of the NHO[double bond, length as m-dash]C H-bond distance from 1.926 A to 1.723 A, equivalent to about a 25% reduction in the HO distance compared to full H-atom transfer. As a result, the excited state process near the S1 origin is a hydrogen atom dislocation that is brought about primarily by heavy atom motion, since the shortened H-bond distance results from extensive heavy-atom motion, with only a 0.03 A increase in the NH bond length relative to its ground state value.
Comparative static and shaking culture of metabolite derived from methyl red degradation by Lysinibacillus fusiformis strain W1B6.[Pubmed:31417722]
R Soc Open Sci. 2019 Jul 31;6(7):190152.
This paper reports on the comparative characteristics and properties of the metabolites derived from methyl red (MR) decolorization by Lysinibacillus fusiformis strain W1B6 under static and shaking conditions. A batch culture system was used to investigate the effect of aeration on azoreductase activity in the biodegradation process, transformation of colour removal and the metabolite products. Biodegradation analysis was monitored using Fourier transform infrared spectroscopy and high-performance liquid chromatography while metabolites were determined using gas chromatography-mass spectroscopy. Phytotoxicity and anti-microbial tests were also conducted to detect the toxicity of metabolites. The results showed that this strain grew more rapidly under shaking conditions while azoreductase activity increased more rapidly under static conditions. Despite that, no significant difference in the decolorization was observed under both static and shaking conditions with up to 96% and 93.6% decolorization achieved, respectively, within 4 h of incubation. MR was degraded into two fragmented compounds, i.e. 2-aminobenzoic acid and N,N-dimethyl-1.4-benzenediamine. The concentration of 2-amino benzoic acid was higher under static conditions resulting the biotransformation of 2-amino benzoic acid into Methyl anthranilate more rapidly under static conditions. Other metabolites were also detected as intermediate biotransformation products and by-products. Less or no toxic effect was found in the metabolite degradation products under both culture conditions.
Synthesis of Methylated Anthranilate Derivatives Using Engineered Strains of Escherichia coli.[Pubmed:31154751]
J Microbiol Biotechnol. 2019 Jun 28;29(6):839-844.
Anthranilate derivatives have been used as flavoring and fragrant agents for a long time. Recently, these compounds are gaining attention due to new biological functions including antinociceptive and analgesic activities. Three anthranilate derivatives, N-methylanthranilate, Methyl anthranilate, and methyl N-methylanthranilate were synthesized using metabolically engineered stains of Escherichia coli. NMT encoding N-methyltransferase from Ruta graveolens, AMAT encoding anthraniloyl-coenzyme A (CoA):methanol acyltransferase from Vitis labrusca, and pqsA encoding anthranilate coenzyme A ligase from Pseudomonas aeruginosa were cloned and E. coli strains harboring these genes were used to synthesize the three desired compounds. E. coli mutants (metJ, trpD, tyrR mutants), which provide more anthranilate and/or S-adenosyl methionine, were used to increase the production of the synthesized compounds. MS/MS analysis was used to determine the structure of the products. Approximately, 185.3 muM N-methylanthranilate and 95.2 muM methyl N-methylanthranilate were synthesized. This is the first report about the synthesis of anthranilate derivatives in E. coli.
Microbial production of methyl anthranilate, a grape flavor compound.[Pubmed:31085637]
Proc Natl Acad Sci U S A. 2019 May 28;116(22):10749-10756.
Methyl anthranilate (MANT) is a widely used compound to give grape scent and flavor, but is currently produced by petroleum-based processes. Here, we report the direct fermentative production of MANT from glucose by metabolically engineered Escherichia coli and Corynebacterium glutamicum strains harboring a synthetic plant-derived metabolic pathway. Optimizing the key enzyme anthranilic acid (ANT) methyltransferase1 (AAMT1) expression, increasing the direct precursor ANT supply, and enhancing the intracellular availability and salvage of the cofactor S-adenosyl-l-methionine required by AAMT1, results in improved MANT production in both engineered microorganisms. Furthermore, in situ two-phase extractive fermentation using tributyrin as an extractant is developed to overcome MANT toxicity. Fed-batch cultures of the final engineered E. coli and C. glutamicum strains in two-phase cultivation mode led to the production of 4.47 and 5.74 g/L MANT, respectively, in minimal media containing glucose. The metabolic engineering strategies developed here will be useful for the production of volatile aromatic esters including MANT.
The MYB transcription factor Emission of Methyl Anthranilate 1 stimulates emission of methyl anthranilate from Medicago truncatula hairy roots.[Pubmed:31009122]
Plant J. 2019 Aug;99(4):637-654.
Plants respond to herbivore or pathogen attacks by activating specific defense programs that include the production of bioactive specialized metabolites to eliminate or deter the attackers. Volatiles play an important role in the interaction of a plant with its environment. Through transcript profiling of jasmonate-elicited Medicago truncatula cells, we identified Emission of Methyl anthranilate (EMA) 1, a MYB transcription factor that is involved in the emission of the volatile compound Methyl anthranilate when expressed in M. truncatula hairy roots, giving them a fruity scent. RNA sequencing (RNA-Seq) analysis of the fragrant roots revealed the upregulation of a methyltransferase that was subsequently characterized to catalyze the O-methylation of anthranilic acid and was hence named M. truncatula anthranilic acid methyl transferase (MtAAMT) 1. Given that direct activation of the MtAAMT1 promoter by EMA1 could not be unambiguously demonstrated, we further probed the RNA-Seq data and identified the repressor protein M. truncatula plant AT-rich sequence and zinc-binding (MtPLATZ) 1. Emission of Methyl anthranilate 1 binds a tandem repeat of the ACCTAAC motif in the MtPLATZ1 promoter to transactivate gene expression. Overexpression of MtPLATZ1 in transgenic M. truncatula hairy roots led to transcriptional silencing of EMA1, indicating that MtPLATZ1 may be part of a negative feedback loop to control the expression of EMA1. Finally, application of exogenous Methyl anthranilate boosted EMA1 and MtAAMT1 expression dramatically, thus also revealing a positive amplification loop. Such positive and negative feedback loops seem to be the norm rather than the exception in the regulation of plant specialized metabolism.


