NorharmanCAS# 244-63-3 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 244-63-3 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
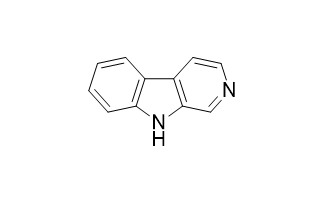
| Formula | C11H8N2 | M.Wt | 168.1 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Norharman is a good inhibitor on MAO A and MAO B isozymes. | |||||

Norharman Dilution Calculator

Norharman Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.9488 mL | 29.7442 mL | 59.4884 mL | 118.9768 mL | 148.721 mL |
| 5 mM | 1.1898 mL | 5.9488 mL | 11.8977 mL | 23.7954 mL | 29.7442 mL |
| 10 mM | 0.5949 mL | 2.9744 mL | 5.9488 mL | 11.8977 mL | 14.8721 mL |
| 50 mM | 0.119 mL | 0.5949 mL | 1.1898 mL | 2.3795 mL | 2.9744 mL |
| 100 mM | 0.0595 mL | 0.2974 mL | 0.5949 mL | 1.1898 mL | 1.4872 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-(4-Hydroxybenzal)acetophenone
Catalog No.:BCN9873
CAS No.:20426-12-4
- Condurango glycoside A
Catalog No.:BCN9872
CAS No.:11051-90-4
- Lappaconine
Catalog No.:BCN9871
CAS No.:23943-93-3
- Cannabiscitrin
Catalog No.:BCN9870
CAS No.:520-14-9
- Datiscin
Catalog No.:BCN9869
CAS No.:16310-92-2
- 15-Methoxy-16-oxo-15,16H-strictic acid
Catalog No.:BCN9868
CAS No.:1356388-38-9
- 1,2-Dimethoxybenzene
Catalog No.:BCN9867
CAS No.:91-16-7
- Tropic acid
Catalog No.:BCN9866
CAS No.:529-64-6
- 4-(4-Methoxyphenyl)-2-butanone
Catalog No.:BCN9865
CAS No.:104-20-1
- ICG-001
Catalog No.:BCN9864
CAS No.:780757-88-2
- 3,29-O-Dibenzoyloxykarounidiol
Catalog No.:BCN9863
CAS No.:389122-01-4
- Somnifericin
Catalog No.:BCN9862
CAS No.:173693-57-7
- Syringetin
Catalog No.:BCN9875
CAS No.:4423-37-4
- 4-Hydroxyisophthalic acid
Catalog No.:BCN9876
CAS No.:636-46-4
- Lyciumamide B
Catalog No.:BCN9877
CAS No.:1647111-41-8
- Tricaprin
Catalog No.:BCN9878
CAS No.:621-71-6
- Dihydroquinine
Catalog No.:BCN9879
CAS No.:522-66-7
- Cymarin
Catalog No.:BCN9880
CAS No.:508-77-0
- 5,7-Dihydroxy 3,3',4',5',6,8-hexamethoxyflavone
Catalog No.:BCN9881
CAS No.:96887-18-2
- gamma-Decalactone
Catalog No.:BCN9882
CAS No.:706-14-9
- 2-(2-Hydroxybenzal)acetophenone
Catalog No.:BCN9883
CAS No.:644-78-0
- (1S,2S,5S)-(-)-Myrtanol
Catalog No.:BCN9884
CAS No.:53369-17-8
- 6-Methoxyflavanone
Catalog No.:BCN9885
CAS No.:3034-04-6
- 15-Deoxypulic acid
Catalog No.:BCN9886
CAS No.:95523-05-0
LC/MS analysis of three-dimensional model cells exposed to cigarette smoke or aerosol from a novel tobacco vapor product.[Pubmed:33268677]
J Toxicol Sci. 2020;45(12):769-782.
A novel tobacco vapor product (NTV) contains tobacco leaves and generates nicotine-containing aerosols using heating elements. Subchronic biological effects have been evaluated previously using three-dimensional bronchial epithelial model cells by repeated exposure to cigarette smoke (CS) and the NTV aerosols; however, the intracellular exposure characteristics have not been studied in detail. In this study, cells were initially exposed to an aqueous extract (AqE) of cigarette smoke (CS) at two concentration levels, and the cell lysate underwent untargeted analysis by LC-high resolution mass spectrometry to determine the exogenous compounds present in the cells. Among the thousands of peaks detected, four peaks showed a CS-dependency, which were reproducibly detected. Two of the peaks were nicotine and nicotine N-oxide, and the other two putative compounds were myosmine and Norharman. The cells were then exposed to an AqE of CS in various combinations of exposure and post-exposure culture durations. Post-exposure culturing of cells with fresh medium markedly decreased the peak areas of the four compounds. The in-vitro switching study of CS to NTV aerosols was investigated by intermittently exposing cells to an AqE of CS four times, followed by exposure to either an AqE of CS, NTV aerosol or medium another four times. Switching to NTV reduced myosmine and Norharman levels, which are known CS constituents. The results indicate that extracellular compounds inside cells reflect the exposure state outside cells. Thus, monitoring functional changes to cells in these exposure experiments is feasible.
Accumulation of heterocyclic amines across low-temperature sausage processing stages as revealed by UPLC-MS/MS.[Pubmed:33233245]
Food Res Int. 2020 Nov;137:109668.
The accumulation of heterocyclic amines (HAs) in low-temperature sausages in each processing stage was investigated using ultra-performance liquid chromatography-mass spectrometry (UPLC-MS/MS). The maximum total levels for free HAs, protein-bound HAs, and all HAs were respectively 1.91 ng/g, 162.91 ng/g and 164.82 ng/g. Harman, Norharman, Glu-P-1, and PhIP accumulated from raw sausages and reached maximum of 50.88 ng/g, 84.59 ng/g, 9.60 ng/g, and 4.69 ng/g after steaming. The highest level of IQ[4,5-b] was 0.36 ng/g found in raw sausages. AalphaC, MeAalphaC, DMIP, and 1,5,6-TMIP were all produced after drying and reached maximum after steaming: 3.25 ng/g, 6.52 ng/g, 0.15 ng/g, and 2.78 ng/g. Additionally, Phe-P-1 reached a maximum of only 0.02 ng/g after drying. MeIQ was generated only after steaming, reaching a maximum of 2.11 ng/g. These results may provide some basis for the inhibition of HAs in meat products through target processing stages.
Heterocyclic aromatic amine level and quality characteristics of selected Harbin red sausages in the northern Chinese market.[Pubmed:33160211]
Meat Sci. 2021 Feb;172:108360.
The heterocyclic aromatic amine (HAA) level and quality characteristics of selected Harbin red sausages in the northern Chinese market and the possible differences between traditional sausages and conventional sausages were evaluated in this study. Four varieties of traditional sausages and four varieties of conventional sausages were selected. Compared to conventional sausages, traditional sausages had lower moisture content and higher hardness (P < 0.05). Twelve HAAs were evaluated and eight HAAs were detected. The total HAA content was as high as 360.73 ng/g. In particular, the contents of Norharman and Harman were much higher than those of the other HAAs (P < 0.05). Additionally, the HAA contents were higher in the traditional sausages than those in the conventional sausages (P < 0.05). Principal component analysis showed that traditional and conventional sausages had a good separation based on the quality characteristics and total HAA level. The results of this study will provide useful information on the industrial production of smoked meat products.
The Inhibitory Effects of Heterotrigona Itama Honey Marinades on the Formation of Carcinogenic Heterocyclic Amines in Grilled Beef Satay.[Pubmed:32858787]
Molecules. 2020 Aug 26;25(17). pii: molecules25173874.
Heterocyclic amines (HCAs) are carcinogenic food toxicants formed in cooked meats, which may increase the risk of cancer development in humans. Therefore, in this study, the effect of stingless bee honey from different botanical origins on the formation of HCAs in grilled beef satay was investigated. HCAs concentration in grilled beef satay was determined by using high performance liquid chromatography (HPLC). In total, six of the most toxigenic HCAs representing aminoimidazo-azaarenes (AIAs) (MeIQx, 4,8-DiMeIQx, and PhIP) and amino carbolines (Norharman, harman, and AalphaC) groups were identified in all the beef samples investigated. A significant reduction in HCAs was observed in grilled beef marinated in honey as compared to beef samples marinated in table sugar (control), in which the reduction of 95.14%, 88.45%, 85.65%, and 57.22% was observed in gelam, starfruit, acacia, and Apis honey marinades, respectively. According to the partial least squares regression (PLS) model, the inhibition of HCAs in grilled beef was shown to be significantly correlated to the antioxidant activity (IC50) of the honey samples. Therefore, the results of this study revealed that the addition of stingless bee honey could play an important role in reducing HCAs in grilled beef.
Inhibitory effects of Portulaca oleracea L. and selected flavonoid ingredients on heterocyclic amines in roast beef patties and Density Function Theory calculation of binding between heterocyclic amines intermediates and flavonoids.[Pubmed:32795783]
Food Chem. 2021 Jan 30;336:127551.
The inhibitory effects of Portulaca oleracea L. (PO) and its flavonoid ingredients on the formation of heterocyclic amines (HAs) in roast beef patties were investigated. Ten HAs were found in control patties, and the total content was 212.73 +/- 7.13 ng/g. With the addition of PO (1%, 5%, and 10%, w/w), HAs decreased by 62.39%, 68.03%, and 73.75%, respectively. The main flavonoid ingredients (rutin, hesperidin, and flavanone) also present a similar inhibitory effect. The Density Function Theory (DFT) methods were adopted to investigate the inhibitory mechanism. These ingredients bonded with the intermediate to block the formation of Norharman. Both experimental and calculated data of the ingredients were analyzed on their HAs inhibitory capacity. Our results provide a novel and valuable strategy to reduce HAs via a low additive level of medicinal and edible plants. And the correlation between experimental and calculated data could be applied to predict the inhibitory ability of inhibitors.
A novel magnetic solid-phase extraction method for detection of 14 heterocyclic aromatic amines by UPLC-MS/MS in meat products.[Pubmed:32791432]
Food Chem. 2021 Feb 1;337:127630.
The current study developed a cheap and effective method for the simultaneous extraction of 14 heterocyclic aromatic amines (HAAs) in food matrix. Core-shell Fe3O4@PDA nanoparticles were constructed and acted as the magnetic solid-phase extraction adsorbent to separate and purify HAAs from meat products for the first time. Then, UPLC-MS/MS technique was employed to identify and quantify the HAAs easily. Fe3O4@PDA nanoparticles were synthesized and characterized successfully. Totally 14 HAAs were completely separated in 19.99 min with good regression coefficients. LODs and LOQs were in the range of 0.013-0.247 ng/g and 0.056-0.803 ng/g, respectively. The intra-day precisions and inter-day precisions were below 9%. Except for IQ[4,5-b], Phe-p-1, PhIP, other 11 types of HAAs (DMIP, 1,5,6-TMIP, IQ, IQx, MeIQ, MeIQx, 7,8-DiMeIQx, AalphaC, MeAalphaC, Harman, Norharman) could acquire relatively high recoveries (71.06%-108.49%). The proposed method was successfully devoted to the evaluation of HAAs levels in 8 commercial meat products to verify the adaptability.
beta-Carbolines in Experiments on Laboratory Animals.[Pubmed:32722000]
Int J Mol Sci. 2020 Jul 24;21(15). pii: ijms21155245.
Some studies have ascribed a protective effect against neurodegenerative diseases to the beta-carbolines harman (H) and Norharman (NH), which occur mostly in coffee and coffee substitutes. We determined the concentrations of beta-carbolines and undesirable compounds (such as acrylamide) in roasted coffee substitute ingredients and found that chicory coffee was optimal. Two in vivo experiments were conducted with seventeen-month-old male Sprague Dawley rats fed a diet with the addition of pure carboline standards in the first stage, and chicory in the second. We observed an increase in the level of H and NH in blood plasma, as well as higher activity of animals in the battery behavioral test, particularly in the second stage. The results of in vitro studies-particularly the level of the expression in brain tissue of genes associated with aging processes and neurodegenerative diseases-clearly show the benefits of a diet rich in beta-carbolines.
Structure characteristics of flavonoids for heterocyclic aromatic amines inhibition using quantitative structure-activity relationship modeling.[Pubmed:32710583]
J Food Biochem. 2020 Sep;44(9):e13390.
The objective of this study was to investigate the structure characteristics of flavonoids that act as inhibitors for heterocyclic aromatic amines (HAAs) formation. Five quantitative structure-activity relationship models for predicting the inhibitory rates of HAAs (Norharman, harman, PhIP, MeIQx, and 4,8-DiMeIQx) were established using selected chemometric parameters (R(2) : 0.591-0.920), and indicated that the hydrophobicity, hydroxyl groups, and topological structure of flavonoids played important roles in the inhibition of HAAs formation. The 5,7-dihydroxyls in meta-position of the A-ring and the 4'-hydroxyl in the B-ring of flavonoids were critical for the inhibitory effects of HAAs, whereas the introduction of 3-hydroxyl and 3-O-glucoside in the C-ring reduced the inhibitory effects. Catechin served as the most effective inhibitor of HAAs followed by luteolin and genistein. The study can bring us a broader idea for controlling the formation of HAAs according to the structure of flavonoids. PRACTICAL APPLICATIONS: Heterocyclic aromatic amines (HAAs) are a class of organic substances with carcinogenic and mutagenic effect formed during the heating process of meat products. The formation of HAAs can be inhibited by adding natural antioxidants such as flavonoids to the meat during pretreatment. This inhibition is influenced by the unique structure of flavonoids. Thus, there has been an increasing demand to exploit the effective HAAs inhibitors from flavonoids by structure characteristics. Our study showed that the inhibitory effect of flavonoids on the formation of HAAs was mainly depended on their hydrophobicity, hydroxyl groups, and topological structure using the multiple QSAR models. Thus, effective HAAs inhibitors can be explored from dietary flavonoids according their structure characteristics.
Simultaneous generation of acrylamide, beta-carboline heterocyclic amines and advanced glycation ends products in an aqueous Maillard reaction model system.[Pubmed:32629331]
Food Chem. 2020 Dec 1;332:127387.
The simultaneous formation of acrylamide; beta-carboline heterocyclic amines (HAs): harmane and Norharmane; and advanced glycation end products (AGEs) (N(epsilon)-(carboxymethyl)lysine (CML) and N(epsilon)-(carboxyethyl)lysine (CEL)) was analyzed based on an aqueous model system. The model systems included lysine-glucose (Lys/Glu), asparagine-glucose (Asn/Glu), tryptophan-glucose (Trp/Glu), and a mixture of these amino acids (Mix/Glu). Only AGEs were generated when heated at 100 degrees C, Asn and Trp competed with Lys for glucose and methylglyoxal (MGO), and glyoxal (GO) decreased AGE content. The k value of CML, CEL, and acrylamide decreased when heated at 130 degrees C, whereas that of harmane increased in the Mix/Glu, owing to the competition between Lys and Asn for glucose, GO, and MGO. Harmane preferably formed via the Pictet-Spengler condensation between Trp and acetaldehyde, which further reduced acrylamide formation via the acrolein pathway.
Effects of amides from pungent spices on the free and protein-bound heterocyclic amine profiles of roast beef patties by UPLC-MS/MS and multivariate statistical analysis.[Pubmed:32527489]
Food Res Int. 2020 Sep;135:109299.
Ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), principal component analysis (PCA) and orthogonal projection to latent structure-discriminant analysis (OPLS-DA) were used to explore the effects of the amide components (capsaicin, sanshoamide, piperine) of pungent spices widely consumed in China (chili pepper, Sichuan pepper and black pepper) on the profiles of 17 heterocyclic amines (HAs) from seven categories in roast beef patties. Four groups of HAs were detected and quantified in both free and protein-bound states: imidazopyridines (DMIP, 1,5,6-TMIP), imidazoquinolines (MeIQ), imidazoquinoxalines (IQx, 4,8-MeIQx) and beta-carbolines (Norharman, harman). Notably, different amounts of added capsaicin, sanshoamide and piperine had significant inhibitory effects on free DMIP, MeIQx, 4,8-DiMeIQx and harman formation in roast beef patties. Additionally, all three amide components significantly inhibited the formation of protein-bound MeIQ, 4,8-DiMeIQx, harman, Norharman and MeAalphaC. In general, amide ingredients inhibited the formation of beta-carbolines and total HAs by approximately 70%. Furthermore, the addition of 0.005% and 0.010% capsaicin suppressed imidazopyridine formation by 44% and 35%, respectively, and 0.015% capsaicin inhibited imdazoquinoxaline formation by 33%. These findings shed light on the effects of amide ingredients on the formation of free and protein-bound HAs.
Effects of polyphosphates and sodium chloride on heterocyclic amines in roasted beef patties as revealed by UPLC-MS/MS.[Pubmed:32428855]
Food Chem. 2020 Oct 1;326:127016.
The effects of sodium tripolyphosphate (TPP), sodium pyrophosphate (PP), and NaCl at different ionic strengths on the formation of heterocyclic amines (HAs) were investigated in roasted beef patties. Six HAs (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine [PhIP], 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline [MeIQx], 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline [4,8-DiMeIQx], 2-amino-3-methylimidazo[4,5-f]quinoline [IQ], 1-methyl-9H-pyrido[3,4-b] indole [harman], and 9H-pyrido[3,4-b] indole [Norharman]) were identified and quantified. The presence of 0.3% and 0.45% PP significantly increased the formation of PhIP (P < 0.05). Different levels of TPP/PP had no effect on MeIQx, 4,8-DiMeIQx, IQ, Norharman, or harman (P > 0.05), but these products increased in the presence of NaCl at three ionic strengths and NaCl + 0.3% and 0.45% TPP/PP (P < 0.05). High hardness and surface temperatures were observed after treatments with NaCl and NaCl + TPP/PP. The increase in these six HAs in beef patties with the addition of polyphosphates and NaCl did not involve changes in pH, but mainly stemmed from higher surface temperatures during roasting.
beta-carbolines norharman and harman in vegetable oils in China.[Pubmed:32364007]
Food Addit Contam Part B Surveill. 2020 Sep;13(3):193-199.
The beta-carbolines Norharman and harman, two heterocyclic aromatic amines with potential mutagenicity, have been determined in vegetable oils. Identification and analysis were carried out by ultra-performance liquid chromatography-triple quadrupole tandem mass spectrometry (UPLC-MS/MS). In 88 samples analysed, the concentrations of Norharman and harman were < LOD to 336.22 ng/g and < LOD to 505.14 ng/g, respectively. A high variability of Norharman and harman levels among different oil types was observed. Sesame-, flaxseed-, sunflower seed-, peanut- and rapeseed oils were most contaminated. Both beta-carbolines were most likely formed during roasting of the oilseeds. Oil consumption, especially of oils obtained after roasting of the seeds, was a major dietary source of the beta-carbolines Norharman and harman. Under existing oil risk factors, this investigation contributes to the unprecedented and essential information for dietary assessments associated with oil consumption.
A natural indole alkaloid, norharmane, affects PIN expression patterns and compromises root growth in Arabidopsis thaliana.[Pubmed:32278957]
Plant Physiol Biochem. 2020 Jun;151:378-390.
Norharmane is an indole alkaloid that can be found in several terrestrial plants, as well as in some dinoflagellates and cyanobacteria. The aim of this study was to focus on the way this metabolite impacts the plant metabolism of the model species Arabidopsis thaliana. This metabolite caused increase of secondary and adventitious roots, as well as torsion, toxic effects, and a decrease in root length. Moreover, Norharmane altered the cellular arrangement, resulting in unfinished cell walls, decreased auxin content and inhibited PIN proteins activity. All the alterations suggest that Norharmane alters polar auxin transport by inhibiting PIN2, PIN3 and PIN7 transport proteins, thus causing a significant inhibitory effect on the growth of A. thaliana seedlings.
Biological significance of aminophenyl-beta-carboline derivatives formed from co-mutagenic action of beta-carbolines and aniline and o-toluidine and its effect on tumorigenesis in humans: A review.[Pubmed:32247557]
Mutat Res. 2020 Feb - Mar;850-851:503148.
Norharman exists in cigarette smoke and cooked foods and is non-mutagenic among Salmonella strains but mutagenic to S. typhimurium TA98 and YG1024 in the presence of S9 mix and aniline and o-toluidine. Co-mutagenesis of beta-carbolines and aniline and o-toluidine occurs through the formation of novel mutagenic aminophenyl-beta-carboline derivatives including 9-(4'-aminophenyl)-9H-pyrido[3,4-b]indole [aminophenylNorharman] (APNH)] and 9-(4'- amino-3'-methylphenyl)-9H-pyrido[3,4-b]indole [aminomethylphenylNorharman] (AMPNH)]. Since humans are often simultaneously exposed to beta-carbolines and aniline and o-toluidine, their effects on humans should be clarified. The most potent of these, APNH, induced both point mutations and small deletions in the liver and colon of gpt delta transgenic mice. Major APNH-induced mutations in the liver occurred at a G:C base pair, suggesting that APNH-DNA adducts (dG-C8-APNH) are potentially involved in these mutations. Furthermore, APNH induced hepatic and colon tumors harboring K-ras, beta-catenin, and Apc mutations in F344 rats, with high incidence. Mutations at G:C base pairs were predominant, similar to those in the in vivo mutation assay using gpt delta mice. Moreover, APNH detected in human urine samples obtained from both healthy volunteers on a normal diet and inpatients receiving parenteral alimentation; therefore, APNH can be considered an endogenous carcinogen contributing to tumorigenesis. Exposure levels of these aminophenyl-beta-carboline derivatives may be lower than those of carcinogenic heterocyclic amines (HCAs) including 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx); however, their health risks in terms of tumorigenesis may be comparable owing to stronger genotoxic effects of APNH rather than HCAs. This review summarized APNH mutagenicity/carcinogenicity, and its in vivo formation. Moreover, the effect on tumorigenesis in humans also discussed.


