Cinnamtannin A2CAS# 86631-38-1 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 86631-38-1 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Red brown powder |
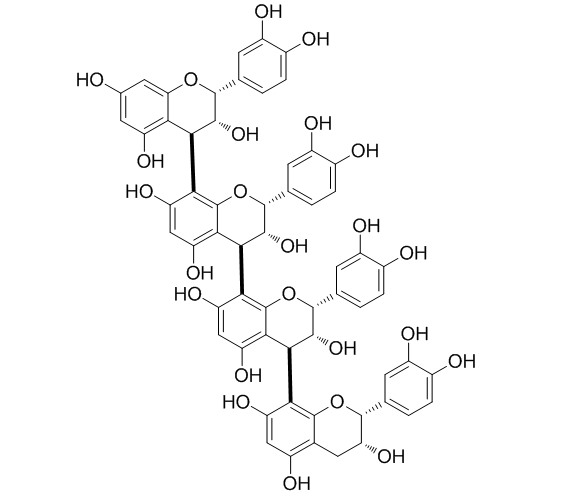
| Formula | C60H50O24 | M.Wt | 1155.1 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Cinnamtannin I; Epicatechin tetramer | ||
| Solubility | Soluble in methanol; sparingly soluble in water | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cinnamtannin A2 may increase the production of mature form SREBPs and LDL receptor activity, thereby increasing ApoA1 and decreasing ApoB levels. | |||||

Cinnamtannin A2 Dilution Calculator

Cinnamtannin A2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.8657 mL | 4.3286 mL | 8.6573 mL | 17.3145 mL | 21.6431 mL |
| 5 mM | 0.1731 mL | 0.8657 mL | 1.7315 mL | 3.4629 mL | 4.3286 mL |
| 10 mM | 0.0866 mL | 0.4329 mL | 0.8657 mL | 1.7315 mL | 2.1643 mL |
| 50 mM | 0.0173 mL | 0.0866 mL | 0.1731 mL | 0.3463 mL | 0.4329 mL |
| 100 mM | 0.0087 mL | 0.0433 mL | 0.0866 mL | 0.1731 mL | 0.2164 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tribulosin
Catalog No.:BCN9888
CAS No.:79974-46-2
- 4-Oxadocosane-1,2-diol
Catalog No.:BCN9887
CAS No.:544-62-7
- 15-Deoxypulic acid
Catalog No.:BCN9886
CAS No.:95523-05-0
- 6-Methoxyflavanone
Catalog No.:BCN9885
CAS No.:3034-04-6
- (1S,2S,5S)-(-)-Myrtanol
Catalog No.:BCN9884
CAS No.:53369-17-8
- 2-(2-Hydroxybenzal)acetophenone
Catalog No.:BCN9883
CAS No.:644-78-0
- gamma-Decalactone
Catalog No.:BCN9882
CAS No.:706-14-9
- 5,7-Dihydroxy 3,3',4',5',6,8-hexamethoxyflavone
Catalog No.:BCN9881
CAS No.:96887-18-2
- Cymarin
Catalog No.:BCN9880
CAS No.:508-77-0
- Dihydroquinine
Catalog No.:BCN9879
CAS No.:522-66-7
- Tricaprin
Catalog No.:BCN9878
CAS No.:621-71-6
- Lyciumamide B
Catalog No.:BCN9877
CAS No.:1647111-41-8
- Tryptamine hydrochloride
Catalog No.:BCN9890
CAS No.:343-94-2
- Caffeoyl alcohol
Catalog No.:BCN9891
CAS No.:3598-26-3
- 5-Geranoxy-7-methoxycoumarin
Catalog No.:BCN9892
CAS No.:7380-39-4
- (-)-Linalool
Catalog No.:BCN9893
CAS No.:126-91-0
- Methyl anthranilate
Catalog No.:BCN9894
CAS No.:134-20-3
- 3-Octanone
Catalog No.:BCN9895
CAS No.:106-68-3
- Decanoic acid
Catalog No.:BCN9896
CAS No.:334-48-5
- 3',4'-Dimethoxyflavone
Catalog No.:BCN9897
CAS No.:4143-62-8
- Dihydroisoferulic acid
Catalog No.:BCN9898
CAS No.:1135-15-5
- cis-Jasmone
Catalog No.:BCN9899
CAS No.:488-10-8
- Methyl benzoate
Catalog No.:BCN9900
CAS No.:93-58-3
- Bufotenine
Catalog No.:BCN9901
CAS No.:487-93-4
Cinnamtannin A2 protects the renal injury by attenuates the altered expression of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) expression in 5/6 nephrectomized rat model.[Pubmed:32385622]
AMB Express. 2020 May 8;10(1):87.
Present investigation determines the protective effect of Cinnamtannin A2 against chronic renal failure (CRF). 5/6 nephrectomized rat model was used to induced CRF by removing the kidneys and rats were treated with Cinnamtannin A2 10 mg/kg, i.p. for the period 30 days. Nephroprotective effect Cinnamtannin A2 was assessed by estimating the biochemical parameters of renal function test and cytokines in the serum of nephractomized rats. Oxidative stress parameters were estimated in the kidney tissue and western blot assay and qRT-PCR assay was performed to determine the expression of protein in renal tissue of nephractomized rats. Moreover histopathology study was done to observe the tubular injury. Data of the report reveals that treatment with Cinnamtannin A2 ameliorates the altered level of creatinine, blood urea nitrogen (BUN), Neutrophil gelatinase-associated lipocalin (NGAL), Kidney Injury Molecule-1 (KIM-1) and cytokines in the serum and microalbuminurea in the urine of 5/6 nephrectomized rat. Oxidative stress level was reduced in Cinnamtannin A2 treated group than CRF group. Moreover treatment with Cinnamtannin A2 attenuates the altered expression of proteins involved in Nrf2-Keap1 pathway in the kidney tissue of 5/6 nephrectomized rat. Result of histopathology reveals that tubular injury score was reduced in the kidney tissue of Cinnamtannin A2 treated group than CRF group. In conclusion, data of the report suggest that treatment with Cinnamtannin A2 ameliorates the level of KIM1 and NAGL in 5/6 nephractomized rats by regulating Nrf2- Keap1 pathway.
Relationship between hemodynamic alteration and sympathetic nerve activation following a single oral dose of cinnamtannin A2.[Pubmed:32321314]
Free Radic Res. 2020 Apr 22:1-25.
We previously found that a single dose of B type procyanidin mixture increase in skeletal muscle blood flow (BF). We compared BF changes following administration of (-)-epicatechin (EC, monomer) and the B type procyanidins procyanidin B2 (B2, dimer), procyanidin C1 (C1, trimer), and Cinnamtannin A2 (A2, tetramer). Each chemical was administered orally to rats, followed by BF measurement in cremaster arteriole for 60 min. 10 and 100 microg/kg of B2 and C1 elicited BF increase, the effect was potent at 100microg/kg. BF also increased significantly after administration of 10 microg/kg A2, but not with the administration at 100 microg/kg. EC yielded no BF changes. Co-treatment with the nonselective adrenaline blocker carvedilol attenuated the BF increase seen with 10 microg/kg A2 treatment. This outcome suggested involvement of sympathetic nerve activation in the BF increase by this dose of A2. Co-treatment of 100 microg/kg A2 with the alpha2 blocker yohimbine exhibited an increase of BF significantly. The alpha2 adrenaline receptor in the vasomotor center is an inhibitory receptor and it regulates hemodynamics. This result suggested that high doses of A2 did not alter BF because of activating the alpha2 adrenergic receptor. Phosphorylation of aortic endothelial nitric oxide synthase (eNOS) increased with 10 microg/kg A2 alone or co-treatment with 100 microg/kg A2 and yohimbine, but not with co-treatment of 10 microg/kg A2 and carvedilol or 100 microg/kg A2 alone. These results imply that A2 does not directly activate eNOS but that shear stress from the increased BF might be associated with eNOS phosphorylation.
Corticotropin-releasing hormone is significantly upregulated in the mouse paraventricular nucleus following a single oral dose of cinnamtannin A2 as an (-)-epicatechin tetramer.[Pubmed:31379411]
J Clin Biochem Nutr. 2019 Jul;65(1):29-33.
Cinnamtannin A2, an (-)-epicatechin tetramer, was reported to have potent physiological activity. Cinnamtannin A2 is rarely absorbed from the gastrointestinal tract into the blood and the mechanisms of its beneficial activities are unknown. Cinnamtannin A2 reported to increase sympathetic nervous activity, which was induced by various stressors. In present study, we examined the stress response in the mouse paraventricular nucleus following a single oral dose of Cinnamtannin A2 by monitoring mRNA expression of corticotropin-releasing hormone (CRH) and c-fos using in situ hybridization. Corticotropin-releasing hormone mRNA showed a tendency to increase at 15 min and significantly increased at 60 min following a single oral administration of 100 microg/kg Cinnamtannin A2. After a single dose of 10 microg/kg Cinnamtannin A2, there was significant upregulation of CRH mRNA at 60 min. These results suggested that Cinnamtannin A2 was recognized as a stressor in central nervous system and this may lead to its beneficial effects on circulation and metabolism.
Absorption, metabolism, distribution and faecal excretion of B-type procyanidin oligomers in mice after a single oral administration of black soybean seed coat extract.[Pubmed:30264089]
Food Funct. 2018 Oct 17;9(10):5362-5370.
We investigated the absorption, metabolism, distribution and faecal excretion of 3 B-type procyanidin oligomers, including procyanidin B2, procyanidin C1 and Cinnamtannin A2, and their monomeric unit (-)-epicatechin after a single oral administration of black soybean seed coat extract (BE) to male ICR mice at 250 mg per kg body weight. Plasma, tissues and faeces samples were collected within 24 h for the determination of (-)-epicatechin, procyandidin B2, procyanidin C1 and Cinnamtannin A2 with or without beta-glucuronidase and sulfatase treatment by the high-performance liquid chromatography method. A portion of the B-type procyanidin oligomers and (-)-epicatechin in BE was absorbed from the small intestine after the oral administration of BE. In the plasma, absorbed procyanidins and (-)-epicatechin existed mainly as conjugates. In the tissues, procyanidin B2, procyandin C1 and Cinnamtannin A2, in addition to (-)-epicatechin distributed widely, primarily in their free forms. Their conjugation occurred mainly in the small intestine, rather than in the liver. Monomeric unit (-)-epicatechin had the highest bioavailability, followed by procyanidin B2, procyanidin C1 and Cinnamtannin A2.
Comparison of the sympathetic stimulatory abilities of B-type procyanidins based on induction of uncoupling protein-1 in brown adipose tissue (BAT) and increased plasma catecholamine (CA) in mice.[Pubmed:30059510]
PLoS One. 2018 Jul 30;13(7):e0201203.
OBJECTIVES: We previously found that elevated energy expenditure following a single oral dose of flavan 3-ols (FL), a mixture of catechins and B type procyanidins, is caused by sympathetic nerve activation. In the present study, we compared the activity of the FL components (-)-epicatechin (EC; monomer), procyanidin B2 (B2; dimer), procyanidin C1 (C1; trimer), Cinnamtannin A2 (A2; tetramer), and more than pentamer fraction (P5). METHODS: Male ICR mice were treated with a single oral dose of FL, EC, B2, C1, A2, or P5. The animals were sacrificed and blood and brown adipose tissue (BAT) sampled. The plasma catecholamine (CA) levels and BAT uncoupling protein (UCP)-1 mRNA expression were determined. RESULTS: A single dose of 10 mg/kg FL significantly increased plasma CA and UCP-1 mRNA levels. B2, C1, and A2, but not EC and P5 (all at 1 mg/kg), significantly increased plasma adrenaline levels. Plasma noradrenaline was significantly elevated by B2 and A2, but not by EC, C1, or P5. UCP-1 mRNA levels were significantly increased by C1 and P5. In the dose response study of A2, 10-3 mg/kg A2 increased UCP-1 mRNA levels significantly, but not 10-2 and 10-1 mg/kg A2. In addition, combination treatment with 10-1 mg/kg A2 and yohimbine, an alpha2 adrenalin blocker, remarkably increased UCP-1 mRNA levels. CONCLUSION: These results suggest that FL and its components, except EC, increase UCP-1 mRNA and plasma CA with varying efficacy.
An analysis method for flavan-3-ols using high performance liquid chromatography coupled with a fluorescence detector.[Pubmed:28911633]
J Food Drug Anal. 2017 Jul;25(3):478-487.
Procyanidins belong to a family of flavan-3-ols, which consist of monomers, (+)-catechin and (-)-epicatechin, and their oligomers and polymers, and are distributed in many plant-derived foods. Procyanidins are reported to have many beneficial physiological activities, such as antihypertensive and anticancer effects. However, the bioavailability of procyanidins is not well understood owing to a lack of convenient and high-sensitive analysis methods. The aim of this study was to develop an improved method for determining procyanidin content in both food materials and biological samples. High performance liquid chromatography (HPLC) coupled with a fluorescence detector was used in this study. The limits of detection (LODs) of (+)-catechin, (-)-epicatechin, procyanidin B2, procyanidin C1, and Cinnamtannin A2 were 3.0x10(-3) ng, 4.0x10(-3) ng, 14.0x10(-3) ng, 18.5x10(-3) ng, and 23.0x10(-3) ng, respectively; the limits of quantification (LOQs) were 10.0x10(-3) ng, 29.0x10(-3) ng, 28.5x10(-3) ng, 54.1x10(-3) ng, and 115.0x10(-3) ng, respectively. The LOD and LOQ values indicated that the sensitivity of the fluorescence detector method was around 1000 times higher than that of conventional HPLC coupled with a UV-detector. We applied the developed method to measure procyanidins in black soybean seed coat extract (BE) prepared from soybeans grown under three different fertilization conditions, namely, conventional farming, basal manure application, and intertillage. The amount of flavan-3-ols in these BEs decreased in the order intertillage > basal manure application > conventional farming. Commercially available BE was orally administered to mice at a dose of 250 mg/kg body weight, and we measured the blood flavan-3-ol content. Data from plasma analysis indicated that up to the tetramer oligomerization, procyanidins were detectable and flavan-3-ols mainly existed in conjugated forms in the plasma. In conclusion, we developed a highly sensitive and convenient analytical method for the analysis of flavan-3-ols, and applied this technique to investigate the bioavailability of flavan-3-ols in biological samples and to measure flavan-3-ol content in food material and plants.
Procyanidin Promotes Translocation of Glucose Transporter 4 in Muscle of Mice through Activation of Insulin and AMPK Signaling Pathways.[Pubmed:27598258]
PLoS One. 2016 Sep 6;11(9):e0161704.
Procyanidins are the oligomeric or polymeric forms of epicatechin and catechin. In this study, we isolated and purified dimer to tetramer procyanidins from black soybean seed coat and investigated the anti-hyperglycemic effects by focusing on glucose transporter 4 (GLUT4) translocation and the underlying molecular mechanism in skeletal muscle of mice. The anti-hyperglycemic effects of procyanidins were also compared with those of monomer (-)-epicatechin (EC) and major anthocyanin, cyanidin-3-O-beta-glucoside (C3G). To investigate GLUT4 translocation and its related signaling pathways, ICR mice were orally given procyanidins, EC and C3G in water at 10 mug/kg body weight. The mice were sacrificed 60 min after the dose of polyphenols, and soleus muscle was extracted from the hind legs. The results showed that trimeric and tetrameric procyanidins activated both insulin- and AMPK-signaling pathways to induce GLUT4 translocation in muscle of ICR mice. We confirmed that procyanidins suppressed acute hyperglycemia with an oral glucose tolerance test in a dose-dependent manner. Of these beneficial effects, Cinnamtannin A2, one of the tetramers, was the most effective. In conclusion, procyanidins, especially Cinnamtannin A2, significantly ameliorate postprandial hyperglycemia at least in part by promoting GLUT4 translocation to the plasma membrane by activating both insulin- and AMPK-signaling pathways.
Extraction of cocoa proanthocyanidins and their fractionation by sequential centrifugal partition chromatography and gel permeation chromatography.[Pubmed:27318471]
Anal Bioanal Chem. 2016 Aug;408(21):5905-5914.
Cocoa beans contain secondary metabolites ranging from simple alkaloids to complex polyphenols with most of them believed to possess significant health benefits. The increasing interest in these health effects has prompted the need to develop techniques for their extraction, fractionation, separation, and analysis. This work provides an update on analytical procedures with a focus on establishing a gentle extraction technique. Cocoa beans were finely ground to an average particle size of <100 mum, defatted at 20 degrees C using n-hexane, and extracted three times with 50 % aqueous acetone at 50 degrees C. Determination of the total phenolic content was done using the Folin-Ciocalteu assay, the concentration of individual polyphenols was analyzed by electrospray ionization high performance liquid chromatography-mass spectrometry (ESI-HPLC/MS). Fractions of bioactive compounds were separated by combining sequential centrifugal partition chromatography (SCPC) and gel permeation column chromatography using Sephadex LH-20. For SCPC, a two-phase solvent system consisting of ethyl acetate/n-butanol/water (4:1:5, v/v/v) was successfully applied for the separation of theobromine, caffeine, and representatives of the two main phenolic compound classes flavan-3-ols and flavonols. Gel permeation chromatography on Sephadex LH-20 using a stepwise elution sequence with aqueous acetone has been shown for effectively separating individual flavan-3-ols. Separation was obtained for (-)-epicatechin, proanthocyanidin dimer B2, trimer C1, and tetramer Cinnamtannin A2. The purity of alkaloids and phenolic compounds was determined by HPLC analysis and their chemical identity was confirmed by mass spectrometry.
Isolation of dimeric, trimeric, tetrameric and pentameric procyanidins from unroasted cocoa beans (Theobroma cacao L.) using countercurrent chromatography.[Pubmed:25722166]
Food Chem. 2015 Jul 15;179:278-89.
The main procyanidins, including dimeric B2 and B5, trimeric C1, tetrameric and pentameric procyanidins, were isolated from unroasted cocoa beans (Theobroma cacao L.) using various techniques of countercurrent chromatography, such as high-speed countercurrent chromatography (HSCCC), low-speed rotary countercurrent chromatography (LSRCCC) and spiral-coil LSRCCC. Furthermore, dimeric procyanidins B1 and B7, which are not present naturally in the analysed cocoa beans, were obtained after semisynthesis of cocoa bean polymers with (+)-catechin as nucleophile and separated by countercurrent chromatography. In this way, the isolation of dimeric procyanidin B1 in considerable amounts (500mg, purity>97%) was possible in a single run. This is the first report concerning the isolation and semisynthesis of dimeric to pentameric procyanidins from T. cacao by countercurrent chromatography. Additionally, the chemical structures of tetrameric (Cinnamtannin A2) and pentameric procyanidins (cinnamtannin A3) were elucidated on the basis of (1)H NMR spectroscopy. Interflavanoid linkage was determined by NOE-correlations, for the first time.
Characterisation of proanthocyanidins from black soybeans: isolation and characterisation of proanthocyanidin oligomers from black soybean seed coats.[Pubmed:23870988]
Food Chem. 2013 Dec 1;141(3):2507-12.
Proanthocyanidin oligomers (dimers to tetramers) were isolated from black soybean seed coats, using Sephadex LH-20 column chromatography and reversed-phase preparative HPLC. The isolated oligomers consisted of only (-)-epicatechin units, which were linked through either 4beta-->8 or 4beta-->6 (B-type) bonds. Procyanidin B2, procyanidin C1, and Cinnamtannin A2 were identified as the main compounds of the proanthocyanidin dimers, trimers, and tetramers, respectively.
Cinnamtannin A2, a tetrameric procyanidin, increases GLP-1 and insulin secretion in mice.[Pubmed:23563558]
Biosci Biotechnol Biochem. 2013;77(4):888-91.
Procyanidins are oligomers and polymers of flavan-3-ols consisting of (-)-epicatechin subunits. In this study, we isolated and purified dimeric, trimeric and tetrameric procyanidins from cacao liquor and investigated their influence on the "incretin effect" as compared to the monomer, (-)-epicatechin in mice. Cinnamtannin A2 specifically increased the glucagon-like peptide-1 (GLP-1) and insulin secretion levels in the plasma after 60 min administration. As evidence of the action of insulin, activation of insulin receptor and insulin receptor substrate-1 was observed in the soleus muscle. These results indicate that the intake of Cinnamtannin A2 may improve hyperglycemia through an incretin-like effect, accompanied by activation of the insulin-signaling pathway.
Cacao polyphenols influence the regulation of apolipoprotein in HepG2 and Caco2 cells.[Pubmed:21226458]
J Agric Food Chem. 2011 Feb 23;59(4):1470-6.
Cocoa powder is rich in polyphenols, such as catechins and procyanidins, and has been shown to inhibit low-density lipoprotein (LDL) oxidation and atherogenesis in a variety of models. Human studies have also shown daily intake of cocoa increases plasma high-density lipoprotein (HDL) and decreases LDL levels. However, the mechanisms responsible for these effects of cocoa on cholesterol metabolism have yet to be fully elucidated. The present study investigated the effects of cacao polyphenols on the production of apolipoproteins A1 and B in human hepatoma HepG2 and intestinal Caco2 cell lines. The cultured HepG2 cells or Caco2 cells were incubated for 24 h in the presence of cacao polyphenols such as (-)-epicatechin, (+)-catechin, procyanidin B2, procyanidin C1, and Cinnamtannin A2. The concentration of apolipoproteins in the cell culture media was quantified using an enzyme-linked immunoassay, and the mRNA expression was quantified by RT-PCR. Cacao polyphenols increased apolipoprotein A1 protein levels and mRNA expression, even though apolipoprotein B protein and the mRNA expression were slightly decreased in both HepG2 cells and Caco2 cells. In addition, cacao polyphenols increased sterol regulatory element binding proteins (SREBPs) and activated LDL receptors in HepG2 cells. These results suggest that cacao polyphenols may increase the production of mature form SREBPs and LDL receptor activity, thereby increasing ApoA1 and decreasing ApoB levels. These results elucidate a novel mechanism by which HDL cholesterol levels become elevated with daily cocoa intake.
Preparative isolation of procyanidins from grape seed extracts by high-speed counter-current chromatography.[Pubmed:18054944]
J Chromatogr A. 2008 Jan 4;1177(1):114-25.
High-speed counter-current chromatography (HSCCC) has been applied to the separation of grape seed procyanidins. The isolation of dimeric to tetrameric procyanidins is achieved after removing the polymeric compounds by solvent precipitation. An additional clean-up by solid-phase extraction on polyamide improved the purities of the isolated compounds. The solvent systems ethyl acetate/2-propanol/water (40:1:40, v/v/v), ethyl acetate/2-propanol/water (20:1:20, v/v/v), and ethyl acetate/1-butanol/water (14:1:15, v/v/v) were successfully used for the fractionation. The combination of HPLC-MS, diode array detection, and NMR analysis, as well as phloroglucinolysis, confirmed the structures of the isolated compounds: B1 [EC-(4beta-->8)-C], B2 [EC-(4beta-->8)-EC], B3 [C-(4alpha-->8)-C], B4 [C-(4alpha-->8)-EC], B5 [EC-4beta-->6-EC], B7 [EC-(4beta-->8)-C], [ECG-(4beta-->8)-C], trimeric procyanidin C1 [EC-4beta-->8-EC-4beta-->8-EC], and the tetrameric procyanidin Cinnamtannin A2 (where C: catechin, EC: epicatechin and ECG: epicatechin-3-O-gallate).


