Zofenopril calciumCAS# 81938-43-4 |
- CEP-32496
Catalog No.:BCC1079
CAS No.:1188910-76-0
- Sorafenib
Catalog No.:BCN2174
CAS No.:284461-73-0
- Vemurafenib (PLX4032, RG7204)
Catalog No.:BCC1269
CAS No.:918504-65-1
- BRAF inhibitor
Catalog No.:BCC1436
CAS No.:918505-61-0
- PLX-4720
Catalog No.:BCC1280
CAS No.:918505-84-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 81938-43-4 | SDF | Download SDF |
| PubChem ID | 3033690 | Appearance | Powder |
| Formula | C44H44CaN2O8S4 | M.Wt | 897.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SQ26991 | ||
| Solubility | H2O : < 0.1 mg/mL (insoluble) DMSO : 2 mg/mL (4.46 mM; Need ultrasonic) | ||
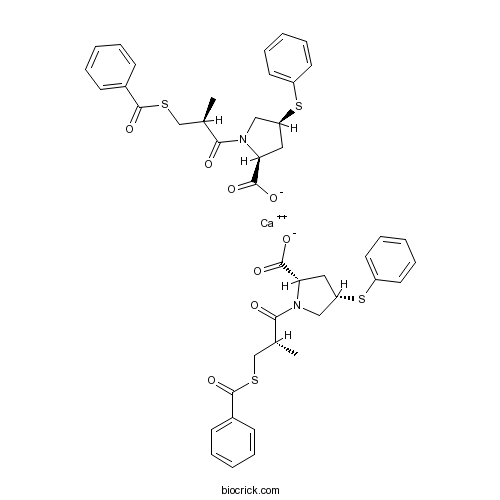
| Chemical Name | calcium;(2S,4S)-1-[(2S)-3-benzoylsulfanyl-2-methylpropanoyl]-4-phenylsulfanylpyrrolidine-2-carboxylate | ||
| SMILES | CC(CSC(=O)C1=CC=CC=C1)C(=O)N2CC(CC2C(=O)[O-])SC3=CC=CC=C3.CC(CSC(=O)C1=CC=CC=C1)C(=O)N2CC(CC2C(=O)[O-])SC3=CC=CC=C3.[Ca+2] | ||
| Standard InChIKey | NSYUKKYYVFVMST-LETVYOFWSA-L | ||
| Standard InChI | InChI=1S/2C22H23NO4S2.Ca/c2*1-15(14-28-22(27)16-8-4-2-5-9-16)20(24)23-13-18(12-19(23)21(25)26)29-17-10-6-3-7-11-17;/h2*2-11,15,18-19H,12-14H2,1H3,(H,25,26);/q;;+2/p-2/t2*15-,18+,19+;/m11./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Zofenopril calcium Dilution Calculator

Zofenopril calcium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1146 mL | 5.5729 mL | 11.1458 mL | 22.2916 mL | 27.8645 mL |
| 5 mM | 0.2229 mL | 1.1146 mL | 2.2292 mL | 4.4583 mL | 5.5729 mL |
| 10 mM | 0.1115 mL | 0.5573 mL | 1.1146 mL | 2.2292 mL | 2.7864 mL |
| 50 mM | 0.0223 mL | 0.1115 mL | 0.2229 mL | 0.4458 mL | 0.5573 mL |
| 100 mM | 0.0111 mL | 0.0557 mL | 0.1115 mL | 0.2229 mL | 0.2786 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Zofenopril Calcium(SQ26991) is an antioxidant that acts as an angiotensin-converting enzyme inhibitor. Target: ACE Zofenopril is a pro-drug designed to undergo metabolic hydrolysis yielding the active free sulfhydryl compound zofenoprilat, which is an angiotensin converting enzyme (ACE) inhibitor [1]. Zofenopril promotes the regeneration of peripheral nerve injuries in rat models [2]. Zofenopril increases SR calcium cycling and stimulates active calcium uptake into the SR [3].
References:
[1]. Dal Bo, L., P. Mazzucchelli, and A. Marzo, Assay of zofenopril and its active metabolite zofenoprilat by liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B Biomed Sci Appl, 2000. 749(2): p. 287-94.
[2]. Kalender, A.M., et al., Effect of Zofenopril on regeneration of sciatic nerve crush injury in a rat model. J Brachial Plex Peripher Nerve Inj, 2009. 4: p. 6.
[3]. Frascarelli, S., et al., Effects of zofenopril on cardiac sarcoplasmic reticulum calcium handling. J Cardiovasc Pharmacol, 2009. 54(5): p. 456-63.
- Polyphyllin H
Catalog No.:BCN2834
CAS No.:81917-50-2
- Momordicoside I aglycone
Catalog No.:BCN4353
CAS No.:81910-41-0
- 5,19-Epoxy-25-methoxycucurbita-6,23-dien-3-ol
Catalog No.:BCN1341
CAS No.:81910-39-6
- Ganoderic acid A
Catalog No.:BCN3033
CAS No.:81907-62-2
- Ganoderic acid B
Catalog No.:BCN3034
CAS No.:81907-61-1
- Forskolin J
Catalog No.:BCN4352
CAS No.:81873-08-7
- Seneciocannabine
Catalog No.:BCN2128
CAS No.:81855-31-4
- Neotriptophenolide
Catalog No.:BCN8059
CAS No.:81827-74-9
- Quinocetone
Catalog No.:BCC9133
CAS No.:81810-66-4
- Methimepip dihydrobromide
Catalog No.:BCC7524
CAS No.:817636-54-7
- Praeruptorin B
Catalog No.:BCN4988
CAS No.:81740-07-0
- Bambuterol
Catalog No.:BCC5431
CAS No.:81732-65-2
- Z-Ligustilide
Catalog No.:BCC9193
CAS No.:81944-09-4
- 4(15),5,10(14)-Germacratrien-1-ol
Catalog No.:BCN4354
CAS No.:81968-62-9
- Splendoside
Catalog No.:BCN6647
CAS No.:81969-41-7
- Clovin
Catalog No.:BCN7817
CAS No.:81970-00-5
- KW-2478
Catalog No.:BCC2127
CAS No.:819812-04-9
- Hyperxanthone E
Catalog No.:BCN8072
CAS No.:819860-76-9
- Vitedoin A
Catalog No.:BCN6741
CAS No.:819861-40-0
- Lancerin
Catalog No.:BCN2803
CAS No.:81991-99-3
- Khellin
Catalog No.:BCN4356
CAS No.:82-02-0
- Benzanthrone
Catalog No.:BCC8845
CAS No.:82-05-3
- Rottlerin
Catalog No.:BCC7127
CAS No.:82-08-6
- Alpha-Toxicarol
Catalog No.:BCN6467
CAS No.:82-09-7
Disposition of zofenopril calcium in healthy subjects.[Pubmed:2292772]
J Pharm Sci. 1990 Nov;79(11):970-3.
Zofenopril calcium (1) is a prodrug that is hydrolyzed in vivo to the active angiotensin-converting enzyme (ACE) inhibitor SQ 26,333 (2). In a two-way crossover study, six healthy male subjects (age range 25-36 years) each received an iv 11.2-mg dose of [14C]SQ 26,703 (14C-3; the L-arginine salt of 2) and an oral 10-mg (equimolar) dose of 14C-1. After the iv dose of 14C-3, the 0-96-h recovery of radioactivity averaged 76 and 16% of the dose in urine and feces, respectively, indicating substantial biliary secretion. After the oral dose of 14C-1, excretion of radioactivity averaged 70% (urine) and 26% (feces). Negligible amounts of 1 were present in urine, indicating complete hydrolysis of the orally administered prodrug. The oral absorption of 1 was almost complete and the oral bioavailability of 2 averaged approximately 70%. The terminal elimination half-life for 2 after the iv dose averaged 5.5 h. Whole body clearance, renal clearance, nonrenal clearance, and Vdss averaged 11.4, 3.1, and 8.3 mL/min/kg and 1.3 L/kg, respectively. These data indicated that 2 is eliminated by the kidney as well as the liver, is extensively metabolized, and is distributed extensively into extravascular sites.
Sites of first-pass bioactivation (hydrolysis) of orally administered zofenopril calcium in dogs.[Pubmed:2052527]
Pharm Res. 1991 Mar;8(3):370-5.
The relative contribution of the gut, liver, and lungs as sites of first-pass bioactivation (hydrolysis) of the orally administered ester prodrug, Zofenopril calcium (SQ 26,991), to the active angiotensin converting enzyme (ACE) inhibitor, SQ 26,333, was determined. With a five-way study design, two dogs each received a single 1.6-mg/kg dose of zofenopril [as its soluble potassium salt (SQ 26,900)] via the following routes of administration: intraarterial, intravenous, intraportal, and oral. Each dog also received an equimolar oral dose of Zofenopril calcium (1.5 mg/kg). Concentrations of zofenopril in plasma were quantitated with a GC/MSD assay. Extraction ratios (E) for zofenopril by the gut, liver, and lungs were calculated based on the ratios of the area under the curve (AUC) values of zofenopril in arterial plasma after administration by the various routes. As individual eliminating organs, the gut and liver each had a high intrinsic capability to hydrolyze zofenopril; E values ranged from 45 to 89%. The lungs were found to have low, but measurable, hydrolytic activity with estimated E values that ranged from 5 to 26%. Overall, about 95% of the orally administered dose of Zofenopril calcium was hydrolyzed during the first pass. Because the prodrug is sequentially exposed to the gut, liver, and lungs, the contribution of the gut to the overall first-pass hydrolysis (ca. 87%) was estimated to be significantly greater than that of the liver (less than 10%) or lungs (less than 2%). Zofenopril was rapidly eliminated after parenteral administration; mean residence time values were 2 min and the elimination half-life values (intraarterial route only) were 9 min.(ABSTRACT TRUNCATED AT 250 WORDS)
Effects of zofenopril on cardiac sarcoplasmic reticulum calcium handling.[Pubmed:19738489]
J Cardiovasc Pharmacol. 2009 Nov;54(5):456-63.
Isolated rat hearts were perfused for 120 minutes in the presence or in the absence of 10 microM zofenoprilat, the active metabolite of zofenopril. At the end of perfusion, cardiac tissue was used to assay sarcoplasmic reticulum (SR) (45)Ca uptake and SR calcium release, which was determined by automatized quick filtration technique after SR vesicle loading with (45)Ca. The expression of genes involved in the control of calcium homeostasis was evaluated by polymerase chain reaction after reverse transcription. In chronic experiments, SR (45)Ca uptake and gene expression were measured in hearts derived from rats treated with 15 mg*kg(-1)*day(-1) zofenopril for 15 days. Acute or chronic zofenopril administration did not produce any change in contractile performance. In acute experiments, SR (45)Ca uptake was significantly increased after exposure to zofenoprilat. The rate constant of calcium-induced calcium release was slightly although not significantly higher, and the calcium leak measured under conditions promoting SR channel closure was significantly increased. In the chronic model, significant increase in the rate of SR (45)Ca uptake was confirmed. Gene expression was not modified, except for decreased phospholamban expression, which is observed in the acute but not in the chronic model. In conclusion, zofenopril increases SR calcium cycling and stimulates active calcium uptake into the SR.
Temperature, pH and agitation rate as dissolution test discriminators of zofenopril calcium tablets.[Pubmed:8003543]
J Pharm Biomed Anal. 1994 Feb;12(2):173-7.
Comparative in vitro dissolution studies were performed on several tablet batches of Zofenopril calcium, an ACE inhibitor, to determine if they could be differentiated on the basis of their release rates. The samples included six batches produced at Site 1 and one batch produced at Site 2. Using regular dissolution conditions (USP paddle method at a 50-rpm agitation speed in phosphate buffer, pH 7.5, at 37 degrees C), release rates of all the tablet batches were similar. By independently altering one of the dissolution test parameters, either a lower pH or a slower agitation rate, discrimination between the Site 1 and Site 2 tablets was enhanced. Discrimination was only slightly enhanced when a lower dissolution medium temperature was used. Tablets made from different polymorphs of Zofenopril calcium could not be differentiated by their dissolution profiles, even with the more discriminating conditions. The dissolution profiles of certain other Zofenopril calcium tablets (including film-coated vs uncoated tablets, and tablets made with micronized vs unmicronized drug particles) were indistinguishable using a 50-rpm agitation rate, but they could be clearly differentiated using a 40-rpm agitation rate.


